Cereal root disease management in Victoria
Author: Grant Hollaway, Joshua Fanning, Frank Henry (Department of Economic Development, Horsham) and Alan McKay (SARDI) | Date: 24 Feb 2015
GRDC project code: DAV00128, DAV00129, DAN00175, DAV00123, DAS00137
Keywords: root lesion nematode, crown rot, cereal cyst nematode, rhizoctonia root rot and take-all.
Take home messages
- Minimise losses associated with root diseases by inspection of plant roots in the previous crop or using a PreDicta B soil test prior to sowing to identify at risk paddocks.
- Crown rot will be an important disease during 2015 if the season finishes with a dry spring as inoculum levels are high from the 2014 season. Reduce risk by rotating to non-cereal crops.
- In paddocks with high numbers of root lesion nematodes, yield losses can be minimised by selecting partially tolerant cultivars and avoiding late sowing. Resistant cultivars can reduce nematode densities and therefore reduce losses in subsequent intolerant crops.
- Cereal cyst nematode is a very damaging nematode if numbers are allowed to increase by growing susceptible cereals.
- Rhizoctonia root rot will likely be a low risk in 2015 if there is a wet summer with multiple rainfall events, provided summer weeds are controlled.
- Take-all will be a low risk in 2015 as the dry spring in 2014 would have limited inoculum build up, while rainfall during January 2015 will have reduced inoculum further.
Background
Cereal root diseases can have serious impacts on grain yield in the absence of adequate control. The key to preventing root diseases is to identify paddocks at risk by inspecting the roots of previous cereal crops or taking a PreDicta B soil test prior to sowing. Knowledge of the potential root diseases in a paddock then enables the most appropriate control strategies to be implemented prior to and/or at sowing. Management must be implemented prior to sowing as there are no in-crop management options available for the control of root diseases, compared with many foliar diseases.
This paper discusses the important root diseases in Victoria, and provides an update on the latest research and an outlook for the 2015 growing season.
Root disease identification and PreDicta B
The simplest way for growers to identify potential root disease risks is to use a PreDicta B soil test prior to sowing. This test identifies and quantifies all of the important soil borne diseases. Recent studies by the Department of Economic Development at Horsham (formerly the Department of Environment and Primary Industries), in collaboration with SARDI, has shown a strong relationship between pre-sowing PreDicta B test results and potential loss in grain yield from both crown rot and root lesion nematodes.
The relationships between pre-sowing crown rot levels, as determined using PreDicta B, and grain yield loss during seasons with both below and above average spring rainfall is shown in Figure 1. This demonstrates how factors such as pre-sowing inoculum level, crop selection and seasonal conditions can all interact to influence the actual loss from disease (Hollaway et al. 2013).
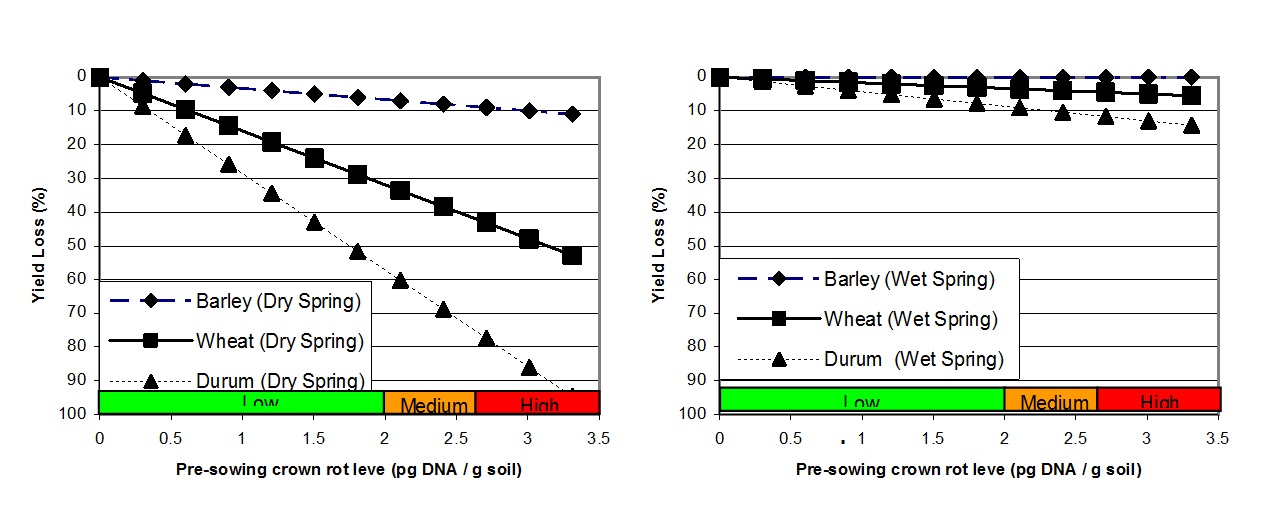
Figure 1. A graphical representation of the relative effect of increasing pre-sowing crown rot inoculum levels, as determined using PreDictaB, on grain yield loss (%) in three cereal crops in seasons with below average (left) and above average (right) combined September and October rainfall using 26 data sets collected in Victoria and South Australia, 2005 to 2010 (Hollaway et al. 2013).
Studies by the Department of Economic Development have shown relationships between pre-sowing root lesion nematode numbers and loss in grain yield (Figure 2). Similar to crown rot the yield loss is related to the tolerance of the crop, but currently our understanding of seasonal effects on yield loss is less well understood.
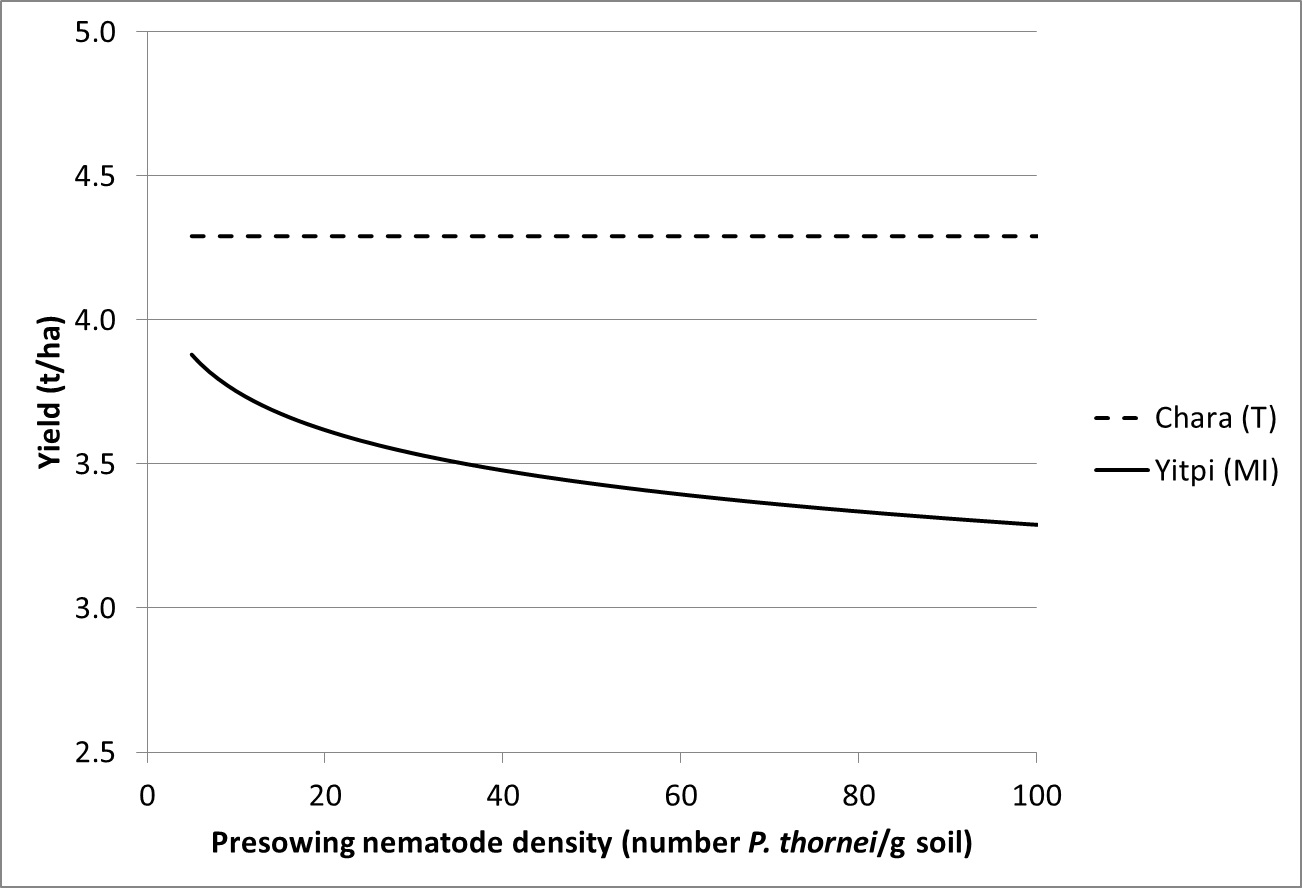
Figure 2. Relationship between grain yield and pre-sowing nematode densities (Pratylenchus thornei) in a tolerant (Chara) and moderately intolerant (Yitpi) wheat cultivar at Banyena in 2011.
Recent studies led by SARDI have re-enforced the need for correct sampling to ensure PreDicta B test results do not underestimate root disease risk. The latest recommendations are to:
- Target sampling along the rows of the last cereal crop.
- Collect three 1x10 cm cores (AccuCore sampling tool) from 15 different locations within the target sampling area/paddock.
- Add one piece of stubble ~5-7 cm long from the base of a cereal plant or grassy weed and add the lower portion to the sample (if present) at each of the 15 sampling locations.
Crown Rot
Crown rot has become a common disease in Victoria, due to the intensification of cereals and stubble retention practices, resulting in losses during seasons with below average spring rainfall (Hollaway and Exell 2010). Losses from crown rot in affected paddocks are usually approximately five per cent, but can also be greater than 20 per cent. In addition to its effects on grain yield, crown rot can also reduce grain size, such as increased screenings.
The distinctive symptom of crown rot is the presence of whiteheads in the crop at early grain fill. These heads mature early and contain no grain or shrivelled grain. Whitehead expression is more common in those seasons with a dry spring. The other, and more reliable, symptom of crown rot is browning of the stem bases. Inspections of stems for browning is best performed from mid to late grain fill through to harvest. To see the browning leaf sheaths should be pulled back and in some cases the pink of the causal fungus may be observed.
Since there is no in-crop control currently available for crown rot it must be controlled prior to sowing. Inspection of previous cereals for the presence of crown rot symptoms provides an indication of potential risk from crown rot. A PreDicta B soil test is also a good option to identify crown rot risk in paddocks that were not previously inspected for symptoms or there was not a cereal crop in the previous year. Figure 1 shows the relationships between pre-sowing inoculum levels and grain yield during seasons with contrasting rainfall (Hollaway et al. 2013).
In paddocks with medium to low crown rot levels, grain yield losses can be minimised by avoiding durum wheat and growing barley in preference to bread wheat (Figure 1 and Table 1). Cereal types varied in yield loss at both Quambatook (trial managed by BCG) and Horsham (Table 1). In addition to comparing cereal type for relative yield loss, two wheat cultivars (Phantom rated MS and Suntop rated MSS to crown rot) with partial resistance to crown rot, were compared with the susceptible wheat cultivar Yitpi(S). These two trials did not show a significant advantage in growing the partially resistant wheats compared with the susceptible wheat. Note that even though barley was a good option for reducing yield loss due to crown rot, it will increase crown rot inoculum levels for a subsequent crop (Figure 3).
Table 1. The effect of crown rot (+) on grain yield of barley, triticale, and bread and durum wheat compared with untreated plots (-) at Quambatook (site managed by the BCG) and Horsham during 2014 with mean yield loss (%) for individual and across sites shown.
| Crop | Variety | Quambatook | Horsham |
Mean yield loss %
(2 sites)B
|
||||
| Grain yield (t/ha)A | Loss (%) | Grain yield (t/ha)A |
Loss (%) | |||||
| - | + | - | + | |||||
| Barley | Hindmarsh | 3.12f |
3.04f |
2 | 4.34f |
4.12ef |
3 | 3 |
| Triticale | Fusion | 1.27e |
1.05d |
18* |
4.03de |
3.91def |
4 | 11 |
| Wheat | Phantom | 0.97cd |
0.89c |
8 | 3.19bc |
2.32a |
27* |
18 |
| Wheat | Yitpi | 1.08d |
0.98cd |
9 | 3.50cd |
2.65ab |
24* |
17 |
| Wheat | Suntop | 1.27e |
1.07d |
16* |
3.50cd |
2.66ab |
24* |
20 |
| Durum | Yawa | 0.72b |
0.39a |
45* |
3.63cde |
2.32a |
36* |
41 |
| Sig Diff | ||
|---|---|---|
| Variety | P<0.001 | P<0.001 |
| Treatment | P<0.001 | P<0.001 |
| Variety x Treatment | P<0.001 | P<0.001 |
| LSD (P=0.05) | ||
| Variety | P<0.001 | P<0.001 |
| Treatment | P<0.001 | P<0.001 |
| Variety x Treatment | P<0.001 | P<0.001 |
| CV(%) | 7.2 | 9.7 |
A Numbers followed by the same letter are not significantly different for that site (P<0.05)
*Significant difference between the crown rot infected and untreated plots for that variety at that site (P<0.05)
In paddocks with high levels of crown rot, it is best to avoid growing cereals. Previous work (Evans et al. 2010) showed the relative effect of cereals (increased inoculum levels) and broadleaf and fallow (decreased inoculum levels) on crown rot inoculum (Figure 3). In general, a 2 year break is required to reduce medium to high inoculum levels to a low level.
Crown rot control: summary
A crown rot management strategy includes:
- testing paddocks prior to sowing to identify risk levels;
- if a paddock has a medium level avoid durum wheat, and consider barley; and
- if a paddock has a high crown rot level, rotate to a grass free break crop for two years.
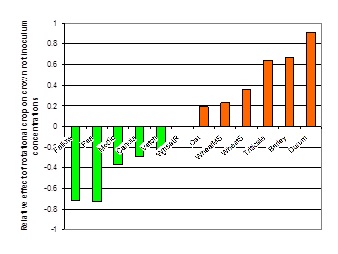
Figure 3. Relative effects of rotational crops (cereal and non-cereals) on crown rot inoculum levels in south eastern Australia, 2003 – 2008 (Evans et al. 2010).
Root lesion nematodes
Root lesion nematodes (RLN) feed on the roots of field crops and can result in yield losses. Although there are many species of root lesion nematodes, the main species in Victoria are Pratylenchus thornei and P. neglectus. They both have a wide host range including crops and weeds. The use of the PreDicta B diagnostic testing service is the best way to identify paddocks with yield limiting densities of root lesion nematodes as this test both quantifies and identifies the species of root lesion nematode.
Yield Loss
During recent years the Department of Economic Development and SARDI have conducted field studies to quantify losses caused by root lesion nematode in the southern cropping region. This work measured grain yield in the presence of high and low numbers of the target nematode. Table 2 shows the average yield loss caused by root lesion nematodes in the five most intolerant cereal cultivars in Victorian field trials. There was large seasonal effects observed. The yield losses caused by P. neglectus were less than those caused by P. thornei. The yield losses observed were smaller than those measured in northern Australia.
Control
Root lesion nematodes can be managed by planting resistant crops or cultivars which reduce nematode densities. Consult the resistance rating in current crop disease guides. There are no currently available chemical control options.
Our recent studies have highlighted the relationship between pre-sowing nematode number and the subsequent nematode multiplication rate for a partially resistant (Hindmarsh) and a susceptible (Yitpi) cereal (Figure 4). This shows how nematode multiplication is influenced by initial nematode number with higher nematode multiplication at lower starting numbers.
Table 2. Average yield loss due to root lesion nematodes in the five most intolerant cereal cultivars across five growing seasons along with average rainfall.| P. thornei (Banyena) | P. neglectus (Dooen) | |||
| Year | Yield Loss (%) | Rainfall (mm) | Yield Loss (%) | Rainfall (mm) |
| 2011 | 12.2 | 241 | 2.0 | 256 |
| 2012 | 9.9 | 268 | 6.7 | 254 |
| 2013 | 1.9 | 353 | 2.5 | 326 |
| 2014 | 4.3 | 253 | 6.7 | 215 |
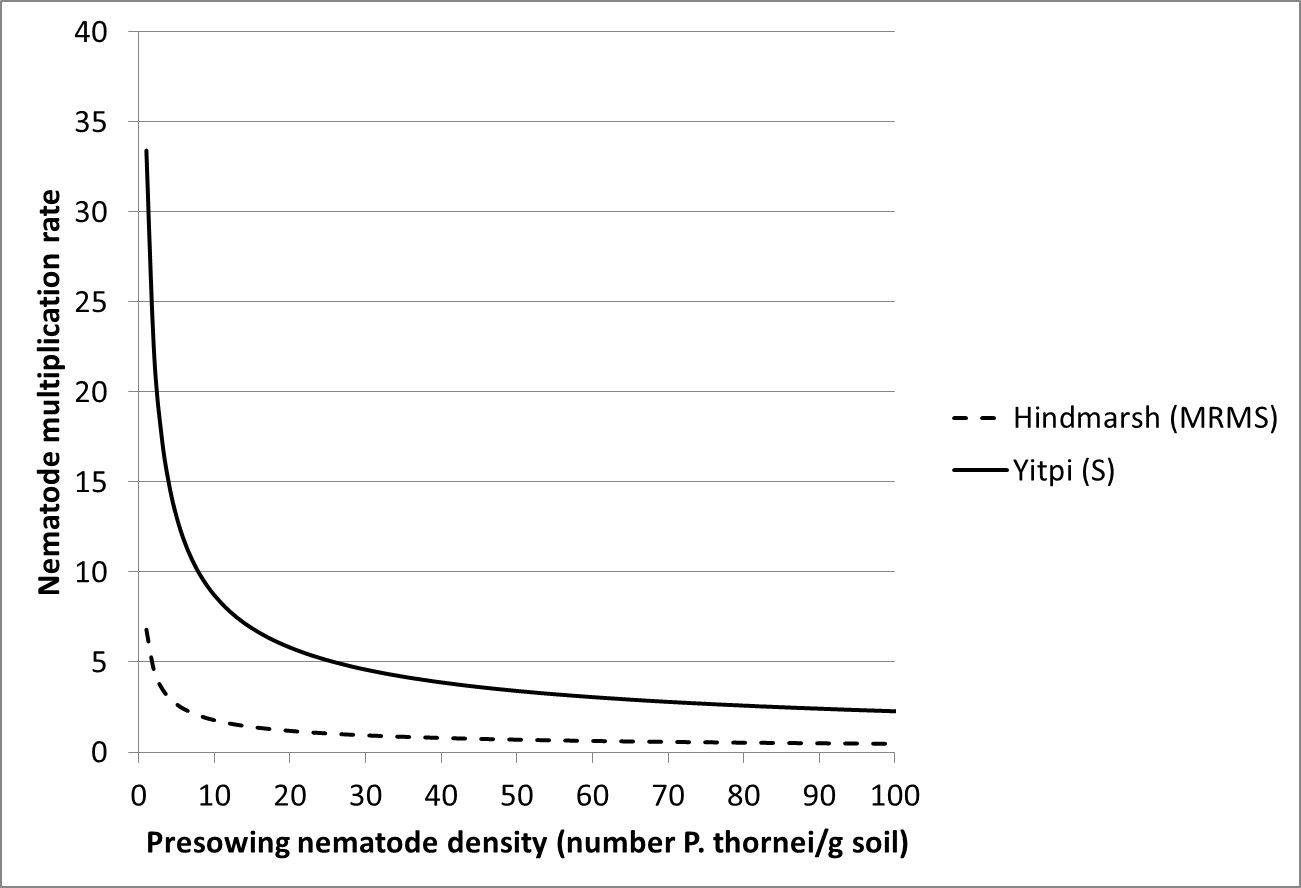
Figure 4. The effect of pre-sowing nematode density on the nematode multiplication rate for a moderately resistant to moderately susceptible (Hindmarsh) and a susceptible (Yitpi) cultivar.
Few cereals are resistant to root lesion nematodes. Crops such as field peas and lentils provide some control options for P. thornei, while faba beans, field peas and lentils provide control options for P. neglectus. Data from trials across the southern region have been collated with provisional resistance ratings established for faba beans, field peas and lentils. These ratings will be reported in the 2015 Victorian cereal and pulse disease guides.
It is important to check the resistance ratings of cultivars as current research is highlighting differences between cultivars even in field pea and lentil cultivars, contrary to previous data. As an example, P. thornei multiplication and provisional resistance ratings are shown in Figure 5 from one field pea trial. This data highlights that some more popular cultivars like Kaspa and PBA Gunyah are not as resistant as other cultivars like PBA Percy and Parafield.
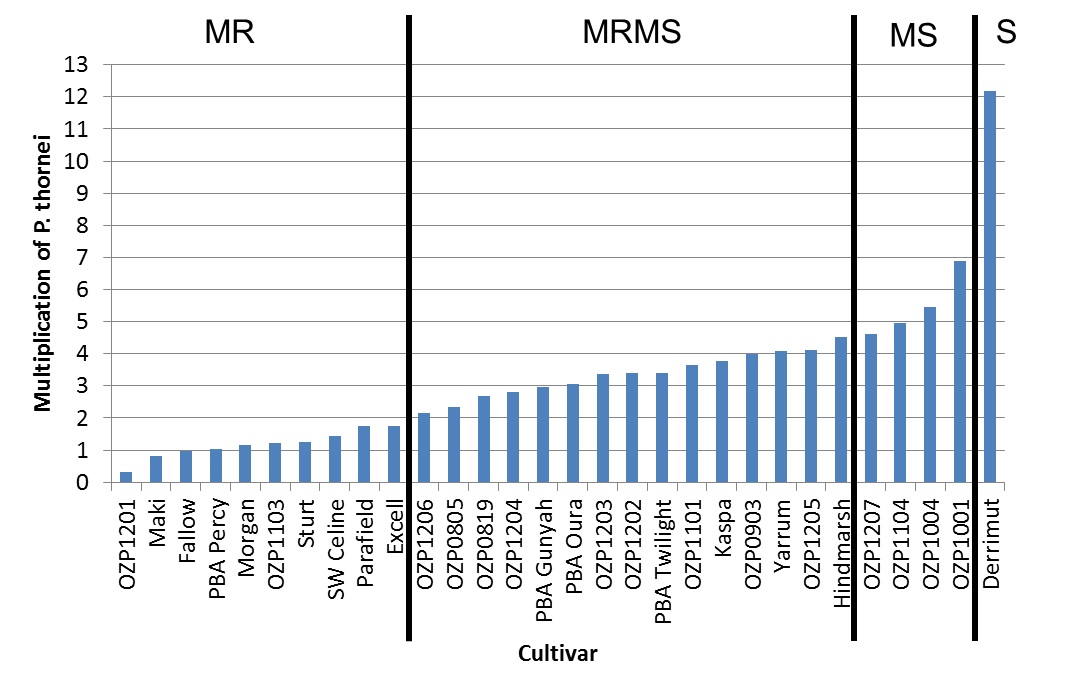
Figure 5. Multiplication rates of Pratylenchus thornei (and provisional resistance ratings) in the presence of field pea cultivars compared to a fallow, susceptible wheat (Derrimut), and a MRMS barley (Hindmarsh).
Cereal cyst nematode
In general, cereal cyst nematode (CCN) has been well managed in Victoria through the widespread use of resistant wheat and barley cultivars (Vanstone et al. 2008). However, farmers need to be aware that growing some new CCN susceptible cultivars will increase CCN levels (Table 3). As show in Table 3, CCN densities quickly increased from 1 to 49 eggs/g of soil within two seasons. As a general guide each egg/g of soil present at sowing equates to approximately one per cent loss in grain yield in intolerant wheat with greater losses also possible.
Should susceptible cereals become common in a rotation, a PreDicta B test should be used to monitor CCN levels. Susceptible cereals should not be grown where CCN is detected. In areas prone to CCN, such as the Wimmera and Mallee, it is important to maintain a high proportion of CCN resistant cereals in the rotation.
Table 3. Effect of susceptible and resistant crops on the density of Cereal Cyst Nematode (CCN) in the Victorian Wimmera during 2010 and 2011.
| CNN Density (CCN/g soil) | |||||
| 2010 | 2011 | ||||
| Start | Treatment | End | Start | Treatment | End |
| 1 | Susceptible | 11 | 11 | Susceptible | 49 |
| Resistant | 3 | ||||
| Resistant | 1 | 1 | Susceptible | 9 | |
| Resistant | 0 | ||||
Rhizoctonia root rot
Symptoms
Plants affected by the Rhizoctonia fungus (Rhizoctonia solani (AG 8)) are usually stunted and sometimes appear purple in colour. If plant roots are severely infected, the root cortex will be 'eaten' away and the central cylinder (stele) will break leaving the characteristic brown 'spear tips'. As a result, bare patches may appear in the crop from an early growth stage when the fungus attacks the seminal roots. Infection of crown roots, later in the season, will result in general unevenness of the crop.
Survival
Soil moisture plays an important role in Rhizoctonia survival. The fungus is capable of growing below permanent wilting point and can survive dry summers. This gives Rhizoctonia a competitive advantage, and disease damage is often severe following dry summers. However, summer rainfall (more than 30 mm in a week) will reduce inoculum as the fungus does not compete well with other soil microorganisms in a warm and moist environment. Multiple rainfall events over summer and autumn can reduce inoculum levels to low risk. Though, where there are long periods between rainfall events (about four weeks) inoculum levels can recover. This can make it difficult to predict the amount of Rhizoctonia inoculum in the soil prior to sowing. Fortunately, a PreDicta B soil test can be used to determine the amount of Rhizoctonia inoculum in the soil and identify high risk paddocks before sowing.
Biology
The Rhizoctonia fungus survives over the summer in plant debris and is generally concentrated in the top 5 to 10 cm of soil. Following the autumn rains, fungal hyphae (threads) grow out of plant debris and spread through the soil, mostly in the horizontal plane, much like a cobweb.
In traditional (conventional) farming systems, cultivation breaks up this fungal cobweb and retards the growth of the fungus reducing Rhizoctonia damage. When farming systems moved to stubble retention and direct drilling there was an increase in the impact of Rhizoctonia because there was less soil disturbance and the stubble provided substrate for the Rhizoctonia fungus.
Research at the time demonstrated it was not necessary to disturb the soil surface if the soil was disturbed 5-10 cm below the seed with a narrow sowing point. This worked because the sowing action breaks up the fungal network in the soil and the deeper cultivation allows the roots systems grow quickly into the soil. As Rhizoctonia can only invade young roots, plants can escape from the fungus before the fungal network grows back.
Further research demonstrated that the high stubble loads in conservation cropping systems encourage the build-up in soil microbes that suppress the activity of Rhizoctonia. This suppressive activity has been shown to increase over a five to eight-year period. So while Rhizoctonia inoculum often increases during the first few years following the adoption of conservation cropping, systems that encourage high soil biological activity, through stubble retention, will build up populations of microbes that suppress the activity of Rhizoctonia, and reduce the seasonal impacts of this disease.
Management
Use a PreDictaD soil test before sowing to identify paddocks that are most at risk of Rhizoctonia.
Control summer and autumn weeds as Rhizoctonia can multiply on the roots of weeds that germinated in response to the rains. Weeds need to be dead for at least 3 weeks before sowing to minimise disease damage.
Use a sowing system with a narrow point that provides soil disturbance 5-10 cm below the seed to break up the Rhizoctonia fungus in the topsoil and allow young roots to grow away from the Rhizoctonia fungus. Disc seeders may not provide enough soil disturbance to reduce the impact of Rhizoctonia.
Consider using a seed treatment that has been reported to supress Rhizoctonia at recommended rates, for example Rancona® Dimension, Dividend M®, VibranceTM, EverGol® Prime or Uniform®.
Avoid sulfonylurea herbicides if possible as they have been shown to increase the severity Rhizoctonia.
Provide adequate crop nutrition, especially zinc as it can reduce the impact of Rhizoctonia.
Take-all
In general take-all should be of less concern during 2015. The dry spring would have limited inoculum build up while the widespread rains during January will have reduced inoculum levels. The most characteristic symptom of take-all is blackening of the sub-crown internode and roots. The take-all fungus infects the centre of the root (stele) so that when roots are snapped and observed end on, the root is back to the core. In severely infected plants this blackening may also progress to the stem base under the leaf sheath.
Conclusion
It is important to actively monitor root disease levels in paddocks so that control strategies can be implemented to prevent in-crop losses.
Acknowledgments
The studies presented in this report were funded by the Victorian Government and the GRDC. Thanks to the cereal pathology technical team (Graham Exell, Mark McLean, Jordan McDonald, Luise Sigel, Tom Pritchett and Melissa Cook) and Adrian Drum for provision of land. Thanks to the BCG for managing the Quambatook crown rot field experiment.
References
- Evans ML, Hollaway GJ, Dennis JI, Correll R, Wallwork H (2010) Crop sequence as a tool for managing populations of Fusarium pseudograminearum and F. culmorum in south-eastern Australia. Australasian Plant Pathology 39, 376-382.
- Hollaway GJ, Evans ML, Wallwork H, Dyson CB, McKay AC (2013) Yield loss in cereals, caused by Fusarium culmorum and F. pseudograminearum (crown rot), is related to concentrations of fungal DNA in soil prior to planting, rainfall and cereal type. Plant Disease 97, 977-982.
- Hollaway GJ, Exell GK (2010) Survey of wheat crops for white heads caused by crown rot in Victoria, 1997-2009. Australasian Plant Pathology 39, 363-367.
- Vanstone VA, Hollaway GJ, Stirling GR (2008) Managing nematode pests in the southern and western regions of the Australian cereal industry: continuing progress in a challenging environment. Australasian Plant Pathology 37 (3), 220-234.
Contact details
Grant Hollaway
Department of Economic Development
Private Bag 260, Horsham Victoria 3401
(03) 5362 2111
grant.hollaway@ecodev.vic.gov.au
GRDC Project Code: DAV00128, DAV00129, DAN00175, DAV00123, DAS00137,
Was this page helpful?
YOUR FEEDBACK
