Understanding wheat phenology: flowering response to sowing time
Author: Felicity Harris (NSW DPI), Peter Martin and Howard Eagles | Date: 16 Feb 2016
Flowering time is critical!
It is critical to match variety and sowing time to ensure flowering occurs during the optimal flowering window to maximise grain yield potential. The optimum flowering window is determined by a balance in water used during canopy development and water used during the grain formation and grain-filling phases (Fischer, 1979), as well as the declining frequency and severity of frosts (Richards, Hunt, Kirkegaard, & Passioura, 2014). Crops that flower too early have increased risk of frost damage, while crops which flower too late have increased risk of high temperatures and water deficit which can restrict grain formation and grain-filling.
How is flowering time regulated?
Accumulated thermal time has a major influence on developmental rate in wheat, with development occurring more rapidly as temperature increases. The relative maturity of varieties is largely controlled by responses to vernalisation and photoperiod.
Vernalisation
Varieties responsive to vernalisation require a period of cold temperatures to make the transition from vegetative to reproductive development. Following saturation of the vernalisation response, floral initiation occurs, marking the completion of the initiation of further leaves and the commencement of the initiation of floral organs at the shoot apex. In wheat, vernalisation accumulates most rapidly between 3-10°C, but can accumulate at a slower rate up to 17°C. Vernalisation can occur at different stages of the individual plant’s lifecycle: during germination, during vegetative plant growth, and for subsequent generations, during seed formation in the mother plant (Flood & Halloran, 1986).
Photoperiod
Wheat is a long-day plant, in which the rate of development is increased with longer day lengths. However, individual genotypes of current commercial varieties have varying levels of responsiveness to photoperiod, including insensitivity. A large number of Australian varieties are insensitive to photoperiod (Cane et al., 2013). In photoperiod sensitive genotypes, short-day (SD) conditions (10 hr or less light) prolong the vegetative phase and delay the transition to reproductive development, whilst long-day (LD) conditions (14 hr or more light) decrease time to floral initiation (Beales, Turner, Griffiths, Snape, & Laurie, 2007).
In vernalisation responsive varieties, following saturation of the vernalisation response, long days hasten progressive inflorescence development and stem elongation. Vernalisation is essentially the prerequisite for long days to reduce the time to flowering (Trevaskis, 2010).
Genetic controls of flowering time
The genetic control of flowering time is complex. There are three primary gene sets with each set controlling vernalisation response, photoperiod response and earliness per se. The genes interact to determine the time of flowering in a specific environment (Slafer & Whitechurch, 2001; Trevaskis, Hemming, Dennis, & Peacock, 2007).
Diagnostic markers for the vernalisation (Vrn-A1, Vrn-B1 and Vrn-D1) and photoperiod (Ppd-B1 and Ppd-D1) genes have enabled characterisation of current commercial varieties (Cane et al., 2013) (Table 1). The traditional classification of winter versus spring wheat has been oversimplified. Identification of the alleles present enabled genotypes to be grouped as either a spring or winter type, or as being photoperiod-sensitive or insensitive. A spring genotype is characterised by the presence of at least one dominant VRN1 allele (a). A winter genotype required recessive VRN1 alleles (v) at the three VRN1 loci in wheat, for example Vrn-A1v + Vrn-B1v + Vrn-D1v (Eagles, Cane, & Vallance, 2009). Photoperiod response is determined by the presence of either the insensitive allele (a), or the sensitive allele (b) at the Ppd-D1 locus (Eagles et al., 2009). A more complex analysis of the effect of these genes on development is made possible by identifying the effect of key alleles and allelic combinations of the known major vernalisation and photoperiod genes. Cane et al. (2013) studied the influence of allelic variation and the interactions between the VRN1 and PPD1 genes, and reported significant differences in heading date attributed to the allelic combinations of Vrn-A1, Vrn-B1, Vrn-D1, Ppd-B1 and Ppd-D1. The identified alleles accounted for 53 per cent of the genetic variance for heading date.
It is likely that other major VRN1 and PPD1 genes affect phasic development in wheat (e.g. Vrn-D4) and that there are other unknown major genes and alleles affecting phasic development. While the general adaptation in flowering time is affected by the major VRN1 and PPD1 genes, flowering time in both winter and spring wheat genotypes is also controlled by a third level of genes, the earliness per se (Eps) genes. The effects of Eps genes are not as defined as vernalisation and photoperiod, and only a few Eps loci have been properly identified in wheat. These have been identified as having more of a fine-tuning effect on flowering time relative to regional adaptations. The interactive nature of the vernalisation and photoperiod genes means the effect of certain genes on flowering time is much greater in specific combinations of alleles.
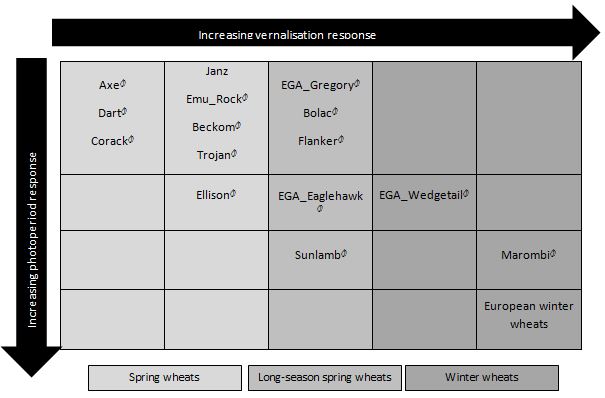
Figure 1: Estimated response of some wheat varieties to vernalisation and photoperiod.
Table 1: The vernalisation and photoperiod genes present in some commercial varieties grown in New South Wales (NSW), their relative maturities and habit classification (Source: Cane et al., 2013).
| Cultivar | Gene* | Maturity** | Type | |||||
|---|---|---|---|---|---|---|---|---|
| Ppd-B1 | Ppd-D1 | Vrn-A1 | Vrn-B1 | Vrn-D1 | ||||
| Axe | a | a | a | a | v | Fast | Spring | |
| Bolac | b | a | a | v | v | Mid-Slow | Spring | |
| Corack | b | a | v | a | a | Mid-Fast | Spring | |
| Dart | b | a | a | a | v | Fast | Spring | |
| EGA_Eaglehawk | b | b | b | v | a | Mid-Slow | Spring | |
| EGA_Gregory | b | a | v | v | a | Mid | Spring | |
| EGA_Wedgetail | b | a | v | v | v | Slow | Winter | |
| Ellison | a | b | v | a | a | Mid-Fast | Spring | |
| Emu_Rock | b | a | a | a | v | Mid-Fast | Spring | |
| Gauntlet |
a | a | a | v | v | Mid-Fast | Spring |
|
| H45 | d | a | a | v | a | Fast | Spring | |
| Harper | b | d | a | a | v | Mid | Spring | |
| Janz | c | a | a | v | v | Mid | Spring | |
| Lancer | a | a | a | v | v | Mid-Slow | Spring | |
| Livingston | b | a | a | a | v | Mid | Spring | |
| Mace | a | a | v | a | v | Mid-Fast | Spring | |
| Marombi | a | b | w | v | v | Slow | Winter | |
| Merlin | b | a | v | a | a | Mid-Fast | Spring | |
| Mitch | b | a | w | a | a | Mid-Slow | Spring | |
| Spitfire | a | a | v | a | a_v | Mid-Fast | Spring | |
| Sunguard | a | a | a | v | v | Mid | Spring | |
| Suntop | d | a | v | a_v | a | Mid | Spring | |
| Sunvale | a | a | a | v | v | Mid | Spring | |
| Trojan | a | c | v | a | a | Mid | Spring | |
*Ppd-B1b is responsive to photoperiod, while Ppd-B1a, Ppd-B1c and PpdB1d are less responsive; Ppd-B1b is a functional gene that is responsive to photoperiod, Ppd-D1a is generally non-responsive to photoperiod and promotes early flowering, while Ppd-D1c and Ppd-D1d are corrupted genes that are largely non-responsive to photoperiod and associated with later flowering; Vrn-A1a and Vrn-A1b are the spring alleles and generally unresponsive to vernalisation, Vrn-A1v is a winter allele and responsive to vernalisation, Vrn-A1w is a winter allele and has a strong vernalisation response; Vrn-B1a and Vrn-D1a are the spring alleles and generally unresponsive to vernalisation, Vrn-B1v and Vrn-D1v are the winter alleles and respond to vernalisation.
**adapted from Cane et al. (2013) and Matthews, McCaffery, and Jenkins (2015). Varieties suited for sowing in NSW range in maturity from slow winter (suited to early sowing) to fast spring types (suited to later sowing).
Flowering response to sowing time
To achieve maximum grain yield potential, it is important to ensure wheat varieties are sown according to their relative maturities (dictated by their response to vernalisation and photoperiod) so flowering occurs in the optimal window.
Wheat varieties responsive to vernalisation (winter types) can be sown early, and will remain vegetative until their vernalisation requirement has been satisfied. This acts to delay reproductive development so that flowering coincides with favourable seasonal conditions. Should a spring variety be sown early (when temperatures are warmer and days longer), development will progress quickly and flowering will occur earlier than optimum, reducing grain yield potential. For example, cv. Dart sown 16 April 2015 at Wagga Wagga flowered on 16 September, while EGA_Wedgetail sown on the same day flowered on 11 October and within the optimum flowering window for Wagga Wagga. However, when sown six weeks later (25 May), they flowered more closely together: Dart flowered 14 October and EGA_Wedgetail flowered 17 October.
In sowing time trials at Wagga Wagga in 2015, the highest grain yields were achieved when flowering occurred at the optimum time (Figure 2). In 2015, there was significant frost damage (around ear emergence) observed in the earliest flowering varieties and below average rainfall in September and October which would have led to moisture stress in the later flowering varieties.
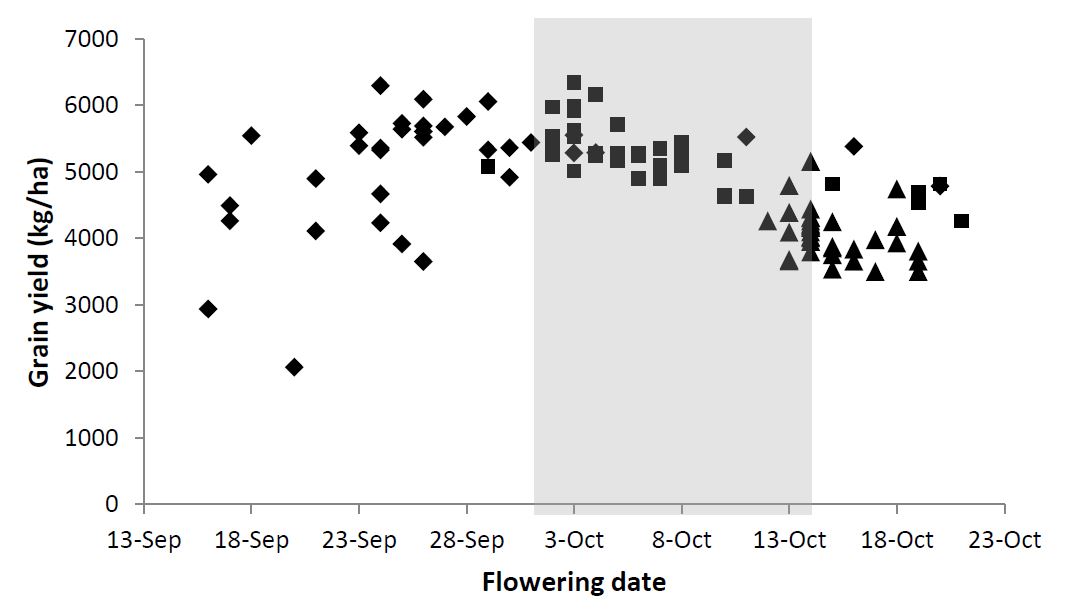
Figure 2: Mean flowering date (DC65) and grain yield for 36 wheat varieties and three sowing times (■ 15 April, (Diamond) 5 May, and ▲ 25 May) at Wagga Wagga, 2015. Shaded area indicates optimal flowering window.
Wheat varieties with varied responses to vernalisation and photoperiod can provide greater flexibility to the sowing schedule. Winter wheats can be sown from late February through to April, long-season spring wheats from mid-April to early May and mid-short season spring wheats from late April onwards. Achieving wheat phenology right by matching sowing time and variety is critical to optimising yield potential, and is low-cost in comparison with other agronomic management tactics.
References
Beales, J., Turner, A., Griffiths, S., Snape, J. W., & Laurie, D. A. (2007). A Pseudo-Response Regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theoretical and Applied Genetics, 115(5), 721-733. doi:10.1007/s00122-007-0603-4.
Cane, K., Eagles, H. A., Laurie, D. A., Trevaskis, B., Vallance, N., Eastwood, R. F., Martin, P. J. (2013). Ppd-B1 and Ppd-D1 and their effects in southern Australian wheat. Crop and Pasture Science, 64(2), 100-114. doi: Ppd-B1 and Ppd-D1 and their effects in southern Australian wheat.
Eagles, H. A., Cane, K., & Vallance, N. (2009). The flow of alleles of important photoperiod and vernalisation genes through Australian wheat. Crop and Pasture Science, 60, 646-657.
Fischer, R. A. (1979). Growth and water limitation to dryland wheat yield in Australia: a physiological framework. The Journal of the Australian Institute of Agriculture, 45, 83-94.
Flood, R. G., & Halloran, G. M. (1986). Genetics and physiology of vernalisation response in wheat. Advances in Agronomy, 39, 87-123.
Matthews, P., McCaffery, D., & Jenkins, L. (2015). Winter Crop Variety Sowing Guide.
Richards, R. A., Hunt, J. R., Kirkegaard, J. A., & Passioura, J. B. (2014). Yield improvement and adaptation of wheat to water-limited environments in Australia—a case study. Crop and Pasture Science, 65(7), 676-689. Yield improvement and adaptation of wheat to water-limited environments in Australia—a case study.
Slafer, G. A., & Whitechurch, E. M. (2001). Chapter 14. Manipulating wheat development to improve adaptation. In M. P. Reynolds, J. I. Ortiz-Monasterio, & A. McNab (Eds.), Application of physiology in wheat breeding (pp. 160-171). Mexico, DF: CIMMYT.
Trevaskis, B. (2010). The central role of the VERNALISATION1 gene in the vernalisation response of cereals. Functional Plant Biology, 37, 479-487.
Trevaskis, B., Hemming, M. N., Dennis, E. S., & Peacock, W. J. (2007). The molecular basis of vernalization-induced flowering in cereals. Trends in Plant Science, 12(8), 352-357. doi:10.1016/j.tplants.2007.06.010.
Contact details
Felicity Harris
felicity.harris@dpi.nsw.gov.au
Was this page helpful?
YOUR FEEDBACK
