Management of yellow spot in wheat: decide before you sow
Author: Steven Simpfendorfer, NSW DPI Tamworth | Date: 05 Mar 2013
Steven Simpfendorfer, NSW DPI Tamworth
Take home message
If you do not want to be concerned by yellow spot in 2013 (including at seedling stages) then:
- DO NOT sow wheat-on-wheat
- If you are going to sow wheat-on-wheat consider a late (autumn) stubble burn and/or
- 3. Select a wheat variety with some level of resistance to yellow spot (note tolerance/resistance to other diseases though).
Primary management decisions for yellow spot need to be made prior to and/or at sowing. Fungicides are a poor last resort for controlling yellow spot as they have reduced efficacy. This IS NOT stripe rust!
Introduction
Yellow spot is stubble-borne leaf disease of wheat which is known by various names such as yellow leaf spot in Western Australia and tan spot in most other countries. Tan spot is arguably a more accurate and less confusing description of the disease.
In recent years some growers and advisers have become confused by the use of the word ‘yellow’ in the disease name and assumed that any yellow discolouration (irrespective if it was associated with a spot or not!) in a wheat crop must be the leaf disease yellow spot and should be sprayed with a fungicide. Consequently, yellow spot was the MOST misdiagnosed wheat disease in the northern region in 2012.
In some situations fungicides were applied to ‘yellow’ wheat crops which were more characteristic of nitrogen deficiency (esp. related to transient waterlogging early in 2012) or herbicide phytotoxicity (especially in tank mixes and when application coincided with frost events). In Queensland it was even reported that frosts during flowering were confused with the disease yellow spot and fungicide applications occurred commercially.
The price of fungicides has dropped dramatically in recent years and they have proved very effective at controlling stripe rust in susceptible wheat varieties when used preventatively and at the appropriate growth stage(s). However, yellow spot is a vastly different disease than stripe rust and fungicides are not a primary option for control due to their reduced efficacy against yellow spot and some key differences in the disease cycle between the two pathogens. The aim of this paper is to provide a plant pathology view of yellow spot and explain some of the reasons why fungicides are NOT a primary tool for the management of yellow spot in wheat.
Yellow spot vs stripe rust
Yellow spot is caused by the fungus, Pyrenophora tritici-repentis and is what is termed a ‘necrotrophic’ pathogen. This simply means that the fungus feeds on dead plant cells. In contrast, stripe rust is caused by a ‘biotrophic’ fungal pathogen which means it feeds off living cells. Why is this difference important?
Being a necrotroph, the yellow spot fungus has to actually kill the wheat cells itself before it can feed on them. To do this the fungus produces a toxin which in the process of killing the cells makes them appear yellow. This is why you get a very discrete yellow margin around a lesion. It is the fungus killing cells just in advance of where it is going to feed next. As the fungus continues to grow in the leaf the lesions get bigger and the previously fed on tissue dries and takes on the characteristic tan (dried dead leaf tissue) appearance. True yellow spot lesions are therefore quite distinct in that they are tan surrounded by a tight yellow margin (halo) where the fungus is producing more toxin to feed next. Logically, it would not be to its advantage to mass produce toxin and kill all the leaf at once, which would appear as more widespread yellowing within leaves, as this would open the tissue up to colonisation by a range of other competing saprophytes (feed off already dead tissue) that are naturally present in every paddock.
Being a necrotrophic leaf pathogen also influences the efficacy of fungicides for logical reasons. Firstly, all fungicides once inside the leaf travel in the xylem and only travel in one direction – towards the leaf tip. As xylem only flows one way being from the base of the leaf towards the tip, the fungicides cannot move back down a leaf. This is why only leaves which have emerged at the time of a fungicide application are protected and the length of protection is dictated by how quick they move in the xylem to the leaf tips.
Stripe rust being a biotroph feeds off living tissue therefore it does not interrupt the xylem activity through its infection process. Mobile DMI fungicides applied to a wheat leaf infected with stripe rust can move through leaf cells and the xylem and effectively kill off the whole infection if applied early enough. DMI fungicides typically have 4-7 days) kick-back activity on stripe rust. This means they can control infection for up to 4-7 days after spores land on the leaf. This is still way before any symptoms of disease can be seen.
In contrast, cells which are killed by the toxin released by the yellow spot fungus include the xylem, so in a tan spot lesion (dried dead leaf tissue) there is no longer xylem activity so there is no way for a fungicide to penetrate this region (remember fungicides only move in xylem). Consequently, fungicides have difficultly accessing an established yellow spot infection to kill it. The process of killing wheat cells in advance of where it feeds gives the yellow spot fungus the added advantage that it also prevents the movement of fungicides into that region of the leaf.
Another key issue is that unlike the longer ‘kick-back’ period for DMI fungicides on a disease like stripe rust, the level of kick back on tan spot is very poor at 0-2 days at best!. For an applied fungicide to control an existing infection, it needs to be applied almost immediately after the infection started. This issue is compounded by potentially continual release of spores from primary and secondary infections on stubble and on the lower leaves of the plant.
Hosts and risk factors
Bread wheat is the primary host of yellow spot but it can also infect durum and triticale but current varieties tend to have moderate levels of resistance. Besides being a pathogen the yellow spot fungus is a very effective saprophyte (it likes to feed on dead tissue!). Hence, as wheat, durum or triticale plants naturally senesce late in the season they become more prone to saprophytic infection irrespective of their resistance rating. Therefore, any wheat variety and for that matter barley, which is not infected when green, can saprophytically host the yellow spot fungus and generate inoculum for the following season. However, as a general rule significantly more inoculum will be generated on stubbles of more susceptible wheat varieties (e.g. EGA Gregory ). Hence, sowing a susceptible wheat variety on stubble of a previously infected susceptible variety (e.g. Gregory-on-Gregory) represents a much higher risk for developing high levels of yellow spot.
). Hence, sowing a susceptible wheat variety on stubble of a previously infected susceptible variety (e.g. Gregory-on-Gregory) represents a much higher risk for developing high levels of yellow spot.
The yellow spot fungus is a stubble-borne pathogen, hence minimum tillage, stubble retention, wheat-on-wheat rotations and growing susceptible wheat varieties (particularly in sequence) dramatically increase the risk of developing damaging levels of yellow spot. However, weather conditions are the overriding risk factor. Yellow spot can develop across a relatively wide temperature range of between 15°C to 28°C being optimal. Greater than 6 hours of leaf wetness or dew are also required for both infections and sporulation (further inoculum production) on old lesions. The incidence and severity of yellow spot therefore increases as moisture periods lengthen such that it takes repeated rain events to drive the disease from the lower to the upper canopy later in the season. Yellow spot is therefore generally considered a significant disease in very wet seasons (e.g. 1998 and 2010) where yield losses of up to 30% have been recorded in susceptible wheat varieties.
Disease cycle
This is covered in great detail with good pictures in a northern region fact sheet produced by GRDC available at http://www.grdc.com.au/GRDC-FS-YellowSpotNorth .
The simplified version is that primary (initial) infections come from microscopic ascospores which are released from black fruiting bodies (pseudothecia) produced on the previous wheat stubble. When the pseudothecia get wet they swell up and eject the ascospores under pressure which allows them to fly less than 10 cm from the stubble. Hence, the initial spread from ascospores off the stubble is only a short distance (metres) which establishes leaf lesions in seedlings. Therefore, seedling infections from yellow spot are largely only in wheat-on-wheat situations.
Secondary spread occurs through production of larger spores called conidia (still microscopic) on initial leaf lesions given adequate moisture. The conidia can be spread further (100 metres) with wind. However, as the secondary conidia are being produced on old lesions in the lower canopy the majority are trapped within the canopy of the current wheat crop and predominantly result in the spread of symptoms from the lower to the upper canopy. Longer distance spread of conidia between paddocks is rare and only a function of particularly wet seasons, such as 1998, where yellow spot was widespread due to the extensive sowing of susceptible varieties and very conducive weather conditions.
The other key point is that yellow spot has a relatively short cycle time (time from spore infection to the development of visible lesions on infected leaves) of between 4-7 days. This is much quicker than the 10-12 days at optimum temperatures with stripe rust. Hence, given a week or two of overcast and rainy weather yellow spot can progress rapidly from old lesions in the lower canopy to affect the upper three main yield contributing leaves.
CORRECT identification: ‘cross off some basics first!’
Yellow spot is frequently misdiagnosed within industry so if you think you have yellow spot or have been advised that you have yellow spot and need to spray a fungicide do some very simple checks first. THINK BEFORE YOU SPRAY!
- Is there wheat stubble visible in the paddock? Yellow spot is essentially a disease of wheat-on-wheat rotations as it is a stubble-borne pathogen. Burning, stubble grazing and cultivation can be effective in reducing yellow spot inoculum but depend on completeness of stubble removal. If wheat stubble is still visible then yellow spot inoculum may also still be present.
- Are black fruiting bodies (pseudothecia) visible on the stubble? By autumn/winter black, pinhead sized, raised structures with hair-like projections (makes them feel rough if rubbed with finger) will be visible on wheat stubble. These are responsible for the initial leaf infections at seedling stages and establishing lesions in lower canopy that drives secondary disease development later in the season.
- Do the lesions ‘SPOTS’ look right? Initially small brown spots with discrete yellow margins which with age become more elongated and tan (dead dried leaf tissue) still with very tight yellow (toxin production) margin. Yellow spot DOES NOT cause extensive general yellowing of leaves.
- Is the distribution both on individual leaves and within leaves on a tiller right? Yellow spot spores (ascospores or conidia) land randomly on individual wheat leaves and just require adequate moisture for >6 h to germinate and infect. Hence, symptoms are randomly distributed across an individual leaf. Yellow spot DOES NOT concentrate towards the leaf tip! Inoculum is generally coming from lower down in the canopy (stubble or old leaf lesions). Hence, if you pull off an individual infected tiller there will be a clear pattern of distribution on the leaves with more and larger lesions on the lowest leaves (they have been out longer so prone to more infection events and greater time for fungal growth to spread through leaf) and fewer and smaller lesions as you progressively move higher up the tiller to the next leaf.
If you cannot comfortably cross each of these boxes then consider getting a second opinion from a plant pathologist. We do have the advantage that we can force the yellow spot fungus to sporulate in the lab and have powerful enough microscopes to observe the distinctive spores to provide a definitive diagnosis, which is not practical in the field.
Why are fungicides less effective against yellow spot?
- The yellow spot fungus is a necrotroph (feeds on dead cells). By killing the wheat cells in advance of where it spreads in a leaf the fungus effectively limits fungicide penetration into the lesion as there is no xylem activity. Hence, a yellow spot lesion is never killed by a fungicide. After fungicide application the production of conidia from the lesions is reduced, but not eliminated, and generally the lesions do not elongate further whilst the active ingredient is still in the leaf (2-4 weeks).
- All fungicides have very limited kick-back activity against yellow spot. Recent German data demonstrates that the main fungicides (and a huge range of experimental actives) only provide between 0-2 days kick-back activity.
- Fungicides have much stronger protectant activity against yellow spot. Again from the German study between 15-30 days at full registered rates of common actives. But when are we applying them in relation to infection events (i.e. rainfall)?
- None of the up-front, seed or in-furrow fungicides have activity against early yellow spot infections.
What about early fungicide sprays?
Yellow spot is commonly a problem in wheat-on-wheat situations at seedling growth stages (emergence through tillering) in the northern region when susceptible varieties are grown (e.g. EGA Gregory , Spitfire
, Spitfire etc). It is less common that yellow spot progresses into the upper canopy to affect the top three leaves which are the main contributors to grain yield as this requires frequent (often prolonged) rain fall events during the season.
etc). It is less common that yellow spot progresses into the upper canopy to affect the top three leaves which are the main contributors to grain yield as this requires frequent (often prolonged) rain fall events during the season.
Recent research data (NGA and GOA) shows that early fungicide applications during these seedling stages are not economical. This is because
- During tillering the key yield contributing leaves (top three) have not emerged yet and will not be protected by a fungicide application at this earlier stage. Fungicides only protect the leaves which they contact and can only move one way towards the leaf tips with the water (xylem) flow.
- Infection from ascospores on the previous wheat stubble can occur over a protracted period as the fruiting bodies do not all mature at the same time. Canadian research has shown that ascospores can be continually released from the stubble from tillering right through to grain-fill (Figure 1). Infected stubble is a source of inoculum throughout the season. New leaves which emerge after a fungicide application are unprotected and can get rapidly infected off the stubble if there is adequate moisture.
- Any inoculum reduction gained through limiting the number of early leaf infections in the lower canopy is quickly swamped by the mass of ascospores released from continually maturing fruiting bodies on the stubble throughout the season, given conducive weather conditions.
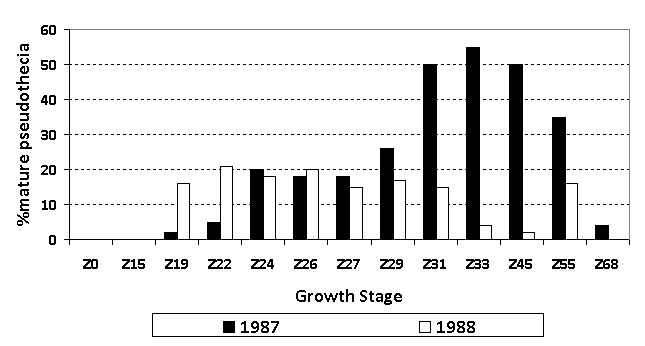
Figure 1: Release of ascospores from yellow spot fruiting bodies on wheat stubble across the season in Canada 1987-1988. Source: Wright et al. (1990) Canadian Journal of Plant Pathology.
Make management decisions at or before sowing
If you do not want to be concerned by yellow spot in 2013 (including at seedling stages) then:
- DO NOT sow wheat-on-wheat
- If you are going to sow wheat-on-wheat consider a late (autumn) stubble burn. Burning, depending on completeness, eliminates the wheat stubble harbouring the yellow spot fungus. A late pre-sow burn, even though cooler, is still preferable to an earlier hotter burn due to soil moisture storage considerations and erosion risk. Cultivation is less advisable as stubble can still remain on the soil surface which carries yellow spot inoculum, it dries out the soil where you are planning to establish your next crop, it reduces water infiltration into the soil (in fallow and throughout season) and it evenly spreads another stubble-borne wheat pathogen, crown rot, across the paddock and distributes it through the soil cultivation layer where it can more easily access the main infection sites in the following cereal crops.
- Select a wheat variety with some level of resistance to yellow spot (note tolerance/resistance to other diseases though especially Pratylenchus thornei).
Primary management decisions for yellow spot need to be made prior to and/or at sowing. Fungicides are a poor last resort for managing yellow spot as they have reduced efficacy against this leaf disease.
Contact details
Steven Simpfendorfer
NSW DPI
Ph: 0439581672
Fx: 0267631222
Email: steven.simpfendorfer@dpi.nsw.gov.au
 Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: DAN00143,
Was this page helpful?
YOUR FEEDBACK
