Optimising the impact of glyphosate
Author: Peter Boutsalis, Gurjeet Gill and Christopher Preston (School of Agriculture, Food & Wine, University of Adelaide) | Date: 24 Feb 2015
GRDC project codes: UA00124, UA00144
Keywords: ryegrass, weeds, glyphosate resistance, weed growth stage, poor application, plant stress, water quality, fitness penalty, resistance testing.
Take home messages
- Glyphosate resistance is occurring in new weed species. Over 500 cases of glyphosate resistant ryegrass have been confirmed in Australia; 78 in Victoria.
- Glyphosate resistance results in reduced efficacy of glyphosate and is dependent on the mechanism of resistance.
- Stressed weeds, poor coverage, poor water quality or dust covered plants can reduce the efficacy of glyphosate.
- Glyphosate efficacy is greater on younger plants and under cooler conditions.
- Using maximum label rates of glyphosate can help overcome factors that may otherwise result in sub-optimum control.
- If glyphosate is used annually or if resistance testing confirms survivors are glyphosate resistant, seed-set control should be implemented to prevent build-up of glyphosate resistant seedbank.
- Resistant testing is recommended to establish if higher rates could be effective.
Glyphosate resistant weed species on the rise
Nationally, the number of species and individual cases of confirmed glyphosate resistance continues to increase. Resistance in new species such as sowthistle and wild radish is of particular concern for Victoria. In Victoria, 78 ryegrass, six windmill grass and two brome populations have been confirmed resistant to glyphosate. A prickly lettuce population from the Wimmera is also suspected to be resistant.
Table 1. Current status of glyphosate resistance in Australia.
| Weed species | Year first documented | No of confirmed cases |
|---|---|---|
| Annual ryegrass (Lolium rigidum) | 1996 | 574 |
| Barnyard grass (Echinochloa colona) | 2007 | 98 |
| Liverseed grass (Urochloa panicoides) | 2008 | 4 |
| Fleabane (Conyza bonariensis) | 2010 | 58 |
| Windmill grass (Chloris truncata) | 2010 | 11 |
| Great brome (Bromus diandrus) | 2011 | 5 |
| Wild radish (Raphanus raphanistrum) | 2013 | 2 |
| Sowthistle (Sonchus oleraceus) | 2014 | 4 |
| Red brome (Bromus rubens) | 2014 | 1 |
Table courtesy of the Chris Preston, Glyphosate Sustainability Working Group
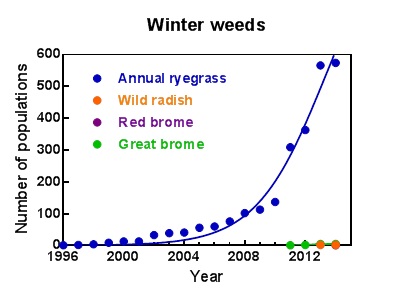
Figure 1. The increase in confirmed cases of glyphosate resistance in winter weeds between 1996 and 2014.
Most cases of glyphosate resistant ryegrass come from samples where weed control had been ineffective. Additionally, in a random weed survey conducted by the University of Adelaide in south eastern Australia in 2013, 16 per cent of the samples were glyphosate resistant. Identification of such high levels of resistance is a serious concern.
Table 2. Glyphosate resistant annual ryegrass has occurred in the following situations.
| Situation | Number of sites | States | |
| Broadacre cropping | Chemical fallow | 32 | NSW |
| Winter grains | 295 | NSW, SA, Vic, WA | |
| Summer grains |
1 | NSW | |
| Irrigated crops |
1 | SA | |
| Horticulture |
Tree crops |
10 | NSW, SA |
| Vine crops | 25 | SA, WA | |
| Vegetables | 2 | Vic | |
| Other | Driveway |
5 | NSW, SA, Vic, WA |
| Fence line / crop margin |
89 | NSW, SA, Vic, WA |
|
| Around buildings | 2 | NSW | |
| Irrigation channel / drain |
14 | NSW, SA, Vic |
|
| Airstrip | 1 | SA | |
| Railway | 2 | NSW, WA | |
| Roadside | 95 | NSW, SA, WA | |
Table courtesy of the Chris Preston, Glyphosate Sustainability Working Group
Reduced efficacy of glyphosate
There are numerous reasons for the poor performance of glyphosate, a common one being herbicide resistance. Resistance to glyphosate can range from weak resistance to strong resistance. Plants with weak resistance may be controlled with higher label rates, but this strategy should not be overused because weeds can develop resistance to very high rates. One Victorian roadside population has survived 20 L/ha glyphosate in pot trials.
Table 3. Percent survival (%) of a selection of grower resistance tests from 2013 and 2014 treated with glyphosate (540g ai/L). Data ranked according to per cent survival at 1000 ml/ha.
| 2013 - Glyphosate 540 (ml/ha) | 2014 - Glyphosate 540 (ml/ha) | ||||||||
| Town | State | 1000 | 1500 | 2000 | Town | State | 1000 | 1500 | 2000 |
| Wagin | WA | 5 | 0 | 0 | Cowangie | Vic | 5 | 5 | 0 |
| Yendon | NSW | 5 | 5 | 0 | Hopetoun | Vic | 5 | 5 | 0 |
| Griffith | NSW | 5 | 5 | 5 | Birchip | Vic | 10 | 0 | 0 |
| Dowerin | WA | 5 | 0 | 0 | Bannockburn | Vic | 10 | 5 | 0 |
| Lake Grace | WA | 10 | 0 | 0 | Dubbo | NSW | 10 | 10 | 5 |
| Yendon | NSW | 20 | 20 | 0 | Deniliquin | Vic | 15 | 5 | 0 |
| Yendon | NSW | 20 | 0 | 0 | Berriwillock | Vic | 15 | 0 | 0 |
| Deniliquin | NSW | 20 | 5 | 0 | Donald | Vic | 20 | 0 | 0 |
| Temora | NSW | 20 | 5 | 0 | Burra | SA | 25 | 25 | 10 |
| Goomalling | WA | 20 | 0 | 0 | Cobram | Vic | 30 | 20 | 0 |
| Goomalling | WA | 20 | 20 | 0 | Birchip | Vic | 30 | 10 | 5 |
| Calingirri | WA | 20 | 0 | 0 | Hopetoun | Vic | 30 | 0 | 0 |
| Griffith | NSW | 20 | 5 | 5 | Hopetoun | Vic | 50 | 20 | 20 |
| Griffith | NSW | 25 | 0 | 0 | Bannockburn | Vic | 50 | 5 | 5 |
| Yendon | NSW | 40 | 20 | 0 | Dubbo | NSW | 50 | 0 | 0 |
| Badgingarra | WA | 50 | 5 | 0 | Donald | Vic | 50 | 50 | 50 |
| Griffith | NSW | 55 | 5 | 5 | Jerrumungup | WA | 60 | 0 | 0 |
| Ballidu | WA | 80 | 70 | 70 | Monarto | SA | 90 | 70 | 40 |
| Griffith | NSW | 80 | 60 | 0 | Quambatook | Vic | 90 | 5 | 0 |
| Nhill | Vic | 80 | 80 | 5 | Echuca | Vic | 100 | 0 | 0 |
| Naracoorte | SA | 90 | 55 | 0 | Bannockburn | Vic | 100 | 0 | 0 |
| Calingirri | WA | 100 | 90 | 90 | Elmore | Vic | 100 | 10 | 0 |
Data courtesy of Peter Boutsalis, Plant Science Consulting
Glyphosate is usually absorbed within 24 hours of application and moves readily in the phloem of actively growing plants. Application of glyphosate in the morning can result in greater uptake than application at night. Greater glyphosate activity is usually observed in actively growing young plants. On larger plants, higher rates are required to maintain good efficacy. Herbicide uptake can be restricted when plants are stressed. Factors that can cause stress include frost, moisture (drought or waterlogging), temperature, nutrition and pest damage. Pot trials have shown that glyphosate activity is often reduced as ambient temperature increases. In ryegrass it has been observed that the optimum daily temperatures for glyphosate activity range between the low teens and mid-twenties. These findings have been observed in unstressed two to three leaved ryegrass growing in pots.
Factors that limit the contact of glyphosate with a target weed include poor coverage (water rates, nozzle selection, applying on very dense populations or wet leaves), poor water quality or application onto dust covered plants. Glyphosate is readily bound to soil particles present as dust or in dirty water.
Applying glyphosate on herbicide resistant weeds that are stressed or exposed to sub-optimum herbicide concentrations (reduced coverage, dust and so on) can result in poor control. Combinations of factors that reduce glyphosate efficacy on plants with weak resistance mechanism can exacerbate the resistance response. Testing for glyphosate resistance using either seed or plants (Quick-Test) can aid in making future weed control decisions. A test can highlight the presence of weak glyphosate resistance mechanisms and if higher rates could be effective under ideal conditions. The identification of strong resistance can aid in convincing growers to adopt alternative strategies to combat the problem.
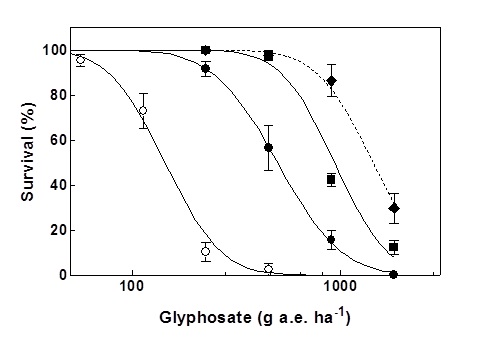
Figure 2. Glyphosate resistance mechanisms are additive. Dose response of ryegrass populations with a target site mutation, SLR 77, (●), the translocation resistance mechanism, NLR 70, (■), and the F1 cross between SLR 77 and NLR 70 (♦) compared with the susceptible population VLR1 (○).
Improving glyphosate efficacy
Using higher label rates can often improve weed control. Weeds with weak glyphosate resistance mechanisms can often be killed with higher label rates. Additionally, higher rates can help counteract poor application, improve control of older plants, stressed plants or overcome reduced efficacy caused by using poor quality water or treating plants covered by dust. Higher label rates can also improve glyphosate activity of plants exposed to higher temperatures that can arise in early autumn or late spring.
Research has shown that although glyphosate resistant weeds are resistant at all growth stages, seedlings are more sensitive than multi-tillered plants. Numerous trials have shown that herbicide resistant weeds are often killed or heavily damaged if treated at the seedling stage. A common strategy by some growers is to delay application of glyphosate to maximise germination from the seedbank in order to 'treat all the weeds'. This strategy can be effective if the weeds are not herbicide resistant or stressed. However, reduced control of older plants that are herbicide resistant can occur if rates are not sufficiently high or weeds are stressed. In weed species that exhibit staggered germination such as brome, wild oats and wild radish, multiple herbicide timings are recommended. The type of resistance mechanism(s) present and more importantly the level of resistance it confers can also influence glyphosate efficacy.
Resources
For more information on herbicide resistance testing visit:
- herbicide resistance seed and quick testing
- crop quality testing
- herbicide resistance seed testing
Acknowledgements
The work presented in this paper was funded by the GRDC.
Contact details
Peter Boutsalis
The University of Adelaide and Plant Science Consulting P/L
0400 664 460
peter.boutsalis@adelaide.edu.au
info@plantscienceconsulting.com.au
Was this page helpful?
YOUR FEEDBACK
