Blackleg – stem/branch cankers and fungicide tolerance
Increased prevalence of blackleg infection of the stems and branches
Blackleg most commonly causes lesions on the cotyledons and leaves of canola plants. It then grows without symptoms through the vascular tissues to the crown where it causes a necrosis resulting in a crown canker at the base of the plant. This crown canker causes yield losses as it restricts water and nutrient uptake by the plant. Blackleg can occur on all plant parts. However, leaf lesions and crown cankers are the most commonly observed symptoms.
In 2010, cankers on the upper stems and branches were observed in a commercial paddock. These cankers appeared to cause yield loss as the pods on affected branches senesced prematurely leading to early pod shatter. Interestingly, preliminary data suggest that stem/branch cankers are not correlated with the presence of traditional crown cankers.
Two species of the Leptosphaeria fungus are present in Australia. The most common species is Leptosphaeria maculans which is more virulent and causes the crown canker symptoms relating to yield loss. Leptosphaeria biglobosa is also present, but is less common. In Europe where L. biglobosa is more widespread, it is associated with damage to the branches. In 2010, isolates were collected from the cankers on the stems and branches, molecular tools were used to genotype these isolates as L. maculans. There was no L. biglobosa identified in any samples.
In 2011, 2012 and 2013 cankers on the stems and branches were observed each year, but symptoms were not generally severe and were not present at all sites. In 2014, the symptoms were widespread and in many cases caused substantial yield loss. In 2015, stem/branch cankers were observed at all sites and again caused severe yield losses in some instances. The presence of effective major gene resistance in cultivars may protect plants from stem/branch cankers.

Figure 1: Symptoms of blackleg infection on flowers, peduncles, upper stems and branches.
Possible causes of stem/branch infection
With the adoption of no-till cropping systems, many canola crops are now sown earlier in the growing season or even into dry soil. These crops are developing earlier, elongating and in many cases flowering in late winter. In the past crops typically remained in the vegetative growth stage during the winter period, elongating at the onset of spring.
Ideal conditions for infection by L. maculans (constant leaf wetness that coincides with ascospore release) occur during winter. This suggests that the stem/branch cankers result from direct infection at the stem elongation/flowering growth stages due to advanced development of the crop.
In 2015, the GRDC/CSIRO canola profitability project (CSP187) had treatments with commercial fungicide applications for blackleg and sclerotinia control applied to crops with different sowing times. Fungicides applied to control blackleg and sclerotinia did not reduce the severity of stem/branch cankers. However, earlier sown crops had significantly greater branch infection than later sown crops (Figure 2).
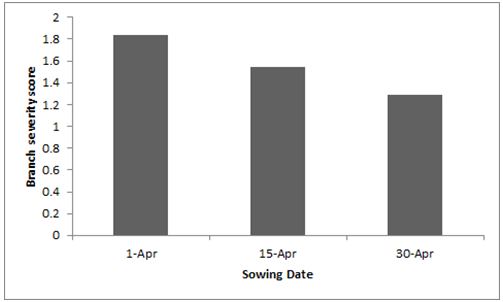
Figure 2: The effect of time of sowing on the severity of blackleg branch infection. (p=0.07, l.s.d. = 0.46). Plants scored on a 0-4 scale (0 being no infection).
Despite the high level of infection, the earlier sowing also had significantly higher yield, which is probably due to the 2015 hot dry spring. Yield may have been even greater in the absence of stem/branch cankers (Figure 3). In a glasshouse experiment, Marcroft Grains Pathology inoculated plants with blackleg spores when plants had elongated 20cm. Plants were treated with various fungicides to determine efficacy in controlling stem cankers. The results are presented in (Table 1).
Table 1: Effect of fungicide application on incidence of plants with a stem/branch lesion after direct infection at the 20cm stem elongation growth stage. Foliar fungicides were applied immediately prior to inoculation.
| Fungicide Treatment | Incidence of plants with a branch/stem lesion |
|---|---|
| Untreated - Range of Foliar fungicides screened |
58% 0-33% |
Management of stem/branch infection
- Try to ensure stem elongation occurs in the normal flowering window, not in winter when blackleg intensity is at its highest
- Reduced blackleg severity by maintaining 500m distance between your crop and the previous season’s stubble.
Blackleg has developed tolerance to fluquinconazole (trade name Jockey®)
In regions where canola is grown intensively growers depend on fungicides to support cultivar resistance. The only commercially available fungicides are different forms of triazoles (DMIs; Group 3). Due to the widespread use of fungicides with the same mechanism of action, and the propensity for L. maculans to overcome cultivar resistance, the canola industry has concerns that L. maculans may develop triazole resistance.
2015 survey
- In 2015 the GRDC provided funding for 200 paddocks to be screened for the presence of fungicide tolerant blackleg populations. This project utilised social media, direct email and the print media to inform growers and advisors of the survey. The project acknowledges the GRDC for their assistance in promoting the survey and getting industry to supply samples from the 200 paddocks to be screened
- Growers and advisers submitted samples from their paddocks which contained 20 randomly selected canola stalks from the previous year’s crop. They also supplied the paddock GPS coordinates, the cultivar sown and fungicides used on the crop
- Cultivar ATR-Stingray was treated with Jockey® at the commercial rate and sown into punnets. Two hundred trays were established, one for each sample to be screened. The untreated seed was used as controls to ensure the inoculation process was successful
- When the seedlings were at the open cotyledon growth stage each individual tray was placed into a single plastic tub. The stubble from the paddock to be screened was placed above the seedlings and wet to trigger ascospore release
- Fourteen days after inoculation each individual plant was scored for the presence of lesions on their cotyledons.
Findings
- Fungicide tolerance to fluquinconazole has been detected for the first time in Australian populations using an in planta assay whereby stubble is used to inoculate seedlings grown from Jockey®-treated seed
- The large-scale survey (200 paddocks) revealed 15 per cent of populations across Australia have this tolerance ranging between seven and 21 per cent for the individual states
- This was the first time a survey has been done, it is unknown whether the frequency of tolerance is increasing, always remained the same or decreasing
- Fluquinconazole still provides some (reduced) protection of the cotyledons (where the fungicide concentration is at its peak), but does not protect older seedlings (where the fungicide has become dilute)
- The fungicide tolerant isolates can cause significant stem cankers at plant maturity
- As yet it is unknown whether there is cross tolerance to other triazole fungicides (in-furrow or foliar).
Table 2: Results of the in planta screen for fungicide tolerance in 200 Australian populations.
| State | Number of samples submitted | Total numbers | Percentage per state |
||
|---|---|---|---|---|---|
| No tolerance | High tolerance | No tolerance | High tolerance | ||
| VIC | 50 | 30 | 7 | 60.0 | 14.0 |
| SA | 44 | 27 | 7 | 61.4 | 15.9 |
| NSW | 62 | 33 | 13 | 53.2 | 21.0 |
| WA | 42 | 32 | 3 | 76.2 | 7.1 |
| ACT | 2 | 1 | 0 | 50.0 | 0.0 |
| Total | 200 | 123 | 30 | 61.5 | 15.0 |

Figure 3: Methodology used for screening Australian blackleg populations for fungicide tolerance. (a) A tray of seedlings are inoculated per population. Two punnets are untreated controls (red circle) whilst the remaining eight punnets are treated with Jockey®. (b) Each tray of seedlings is place under moisten stubble in a plastic tub. (c) All tubs are placed in a constant temperature room for 36 hours to allow spore release to occur. (d) Examples of a ‘no tolerant’ (left) and ‘tolerant’ (right) population.
Recommendations
Fungicide is only one control mechanism against blackleg, it is recommended that growers read the GRDC’s current Blackleg Management Guide available and follow the management steps to reduce the effect of blackleg, such as choosing resistant cultivars, avoiding previous year’s stubble and rotating cultivars to a different resistance group.
Useful resources
GRDC Blackleg Management Guide Fact Sheet
Contact details
Stephen Marcroft
steve@grainspathology.com.au
Was this page helpful?
YOUR FEEDBACK
