How much can pulse agronomy affect the amount of nitrogen fixed
Author: Nikki Seymour (1), RCN Rachaputi (2), Kerry McKenzie (1), Stephen Krosch (1) | Date: 20 Jul 2016
1Department of Agriculture and Fisheries, Queensland
2Queensland Alliance of Agriculture and Food Innovation
Take home message
The amount of N fixed by a pulse crop is largely influenced by how well that crop grows. More crop biomass =more N fixed by that crop provided it is well nodulated.
The amount of N fixed by a legume does not equal the amount available for the next crop. N is removed in the harvested grain and that N remaining in the crop residue then needs to be mineralised by microbial activity before it is available to the next crop.
Row spacing in chickpeas of 0.25 lead to greater biomass, yield and increased N fixation than at 1.0m. N fixation in mungbeans is also significantly better at narrower row spacing particularly in recent varieties.
High soil N levels can significantly reduce N fixation.
Sowing at the optimum time for maximum crop biomass leads to greater amounts of N fixed.
Background
Average amounts of N fixed annually by crop and pasture legumes are around 110 kg N/ha (ranging from close to zero to more than 400kg N/ha). The actual amount fixed depends on the species of legume grown, the site and the seasonal conditions as well as agronomic management of the crop or pasture. The legume crop uses this N for its own growth and may fix significantly more than needed, leaving a positive N balance in the soil for proceeding crops.
Chickpeas were the most widely grown legume crop in Australia in 2013 with about 85 per cent being grown in NSW and Queensland. Mungbeans in the years 2008 - 2010 were up to 70000 t average production after an average of just 40000 t in 2004 -2007, mainly through improved average yields. The vast majority are grown in the northern region of Australia.
The amount of N fixed by a legume increases as legume biomass increases but is reduced by high levels of soil nitrate. In general, legume reliance on N fixation is high when soil nitrate levels are below 50 kg N/ha in the top metre of soil. Above 200 kg N/ha, nitrogen fixation is generally close to zero. The fixed N is used for the growth of the legume itself (saving fertiliser application of the legume crop) as well as potentially leaving residual N for the following cereal or oilseed crop and providing a break from cereal stubble and soil-borne diseases.
Work by Doughton et al. (1993) clearly demonstrated the impact of increasing soil nitrate levels on N fixation of chickpeas (see Figure 1), with no yield advantage being gained by applying N. Moreover, chickpea provided a positive soil N balance when fixation rates were high and a negative balance at low fixation rates.
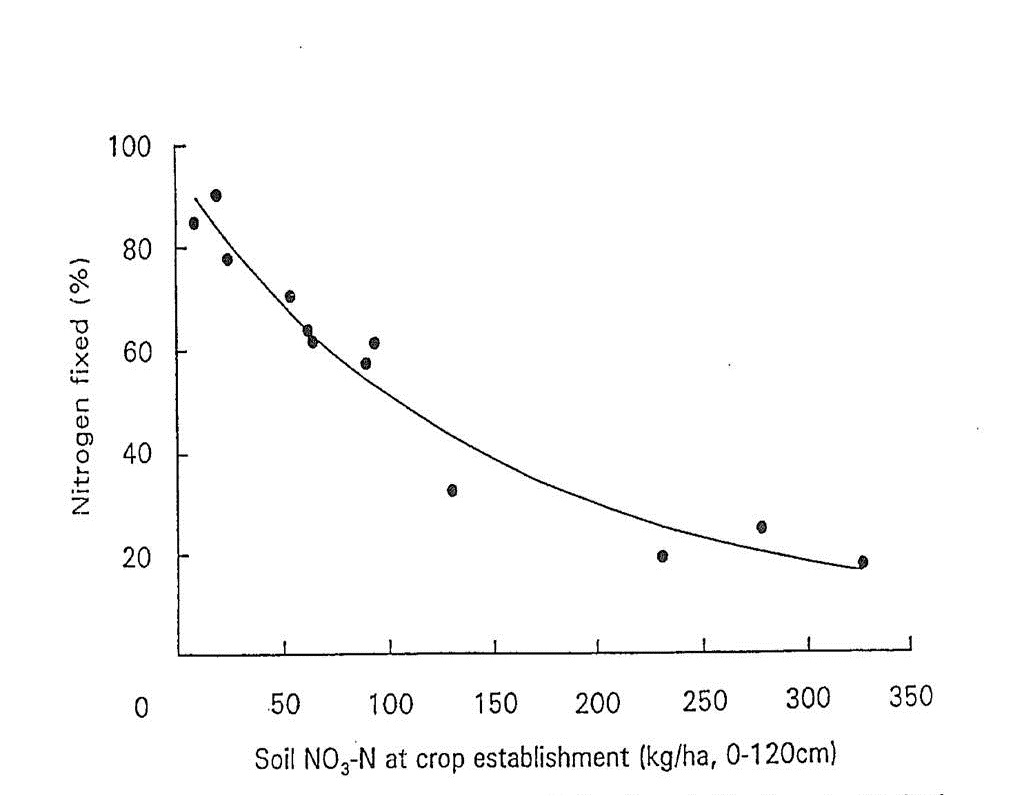
Figure 1. Per cent nitrogen fixed in chickpea (cv. Reselected Tyson) tops 130 days after planting for various levels of soil NO3-N at crop establishment. For fitted curve, Y = 7.05+88.45e-0.0070X, R2 = 0.95 (from Doughton et al. 1993).
Agronomy influences N fixation
The impacts of varying agronomic practices on N fixation are being assessed on trials grown in different environments under the GRDC Pulse Agronomy Initiative in the northern region.
Row spacing
Two chickpea trials conducted recently on the Downs (near Goondiwindi and Dalby) were assessed for the amount of N fixation at different row spacings (all at the same plant population of 30 plants/m2.) Yields were considerably lower at the Goondiwindi site (ranged from 1.5 to 2.1 t/ha) compared to the Dalby site (from 3.2 to 4.7 t/ha) due largely to better seasonal conditions at Dalby, and there was a significant site x genotype x row spacing interaction. Generally, yield and biomass production were reduced as row spacing increased but the amount differed for each genotype. There were no differences in N fixation amongst varieties but row spacing significantly decreased the percent of N fixation (also called %N derived from the atmosphere or %Ndfa) and the total amount of N fixed, particularly at the Dalby site (Table 1).
Up to 59 kg N/ha remained at the Dalby site when chickpeas were grown on 0.25m rows but only 23 kg N/ha from the 1.0 m row spacing. At Goondiwindi, the net N balance ranged from 6 down to -6 kg N/ha as row spacing increased from 0.25m to 1.0m. As a crops’ demand for N increased so did the N fixation by that crop as seen at our Dalby trial grown under better seasonal conditions.
Table 1. Reduction in biomass, N fixation (%N derived from the atmosphere or %Ndfa) and total amount of N fixed in chickpea (meaned across 3 genotypes) as row spacing in the field increases.
|
Shoot dry weight (t/ha) |
%Ndfa |
Total crop N fixed (kg/ha) |
N balance (kg/ha) |
|||||
|
Row spacing (m) |
Dalby |
Goondi |
Dalby |
Goondi |
Dalby |
Goondi |
Dalby |
Goondi |
|
0.25 |
9.89 |
4.75 |
61.0 |
39.2 |
187.3 |
62.8 |
59 |
6 |
|
0.5 |
9.25 |
4.23 |
55.8 |
33.9 |
161.9 |
48.0 |
45 |
-5 |
|
1.0 |
7.96 |
3.59 |
47.6 |
35.4 |
122.5 |
42.0 |
23 |
-6 |
|
LSD (P=0.05) |
1.12 |
0.67 |
7.0 |
n.s. |
31.5 |
16.3 |
n.s. |
n.s. |
In trials with mungbeans in Qld, differences in the amount of nitrogen fixed was evident between varieties and the row spacings across all varieties (Figure 2).
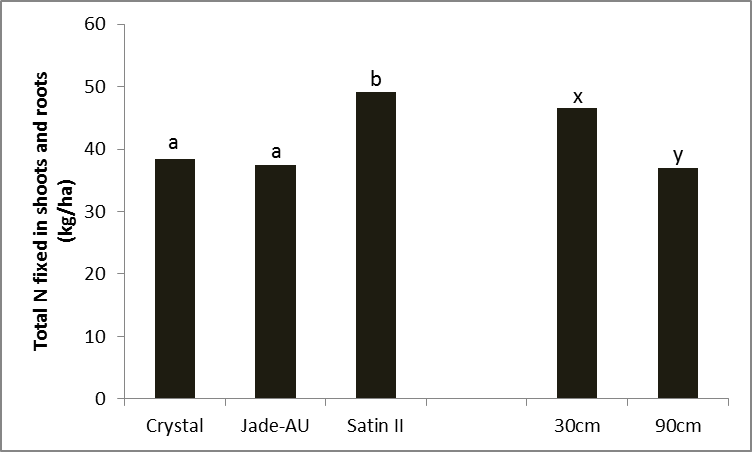
Figure 2. Differences in total shot and root nitrogen by variety (LSD 5% = 7.65) and row spacing (LSD 5% = 6.24), Kingaroy 2012/13
The differences in the amount of N in the shoots and roots (Figure 2) can be influenced by the amount of total dry matter produced or the percent of nitrogen derived from the atmosphere (%Ndfa). It can be seen in Figure 3 that the amount of nitrogen derived from the atmosphere for Crystal and Jade-AU was different with changes in row spacings, however Satin II kept the amount of N from the atmosphere constant at the varying row spacings.
As we have shown with the other trial results narrower rows are producing higher yields which must be supported by higher dry matter production. The crop then has a higher nitrogen demand that is being met by an increase in the nitrogen fixed by rhizobia and provided to the plant.
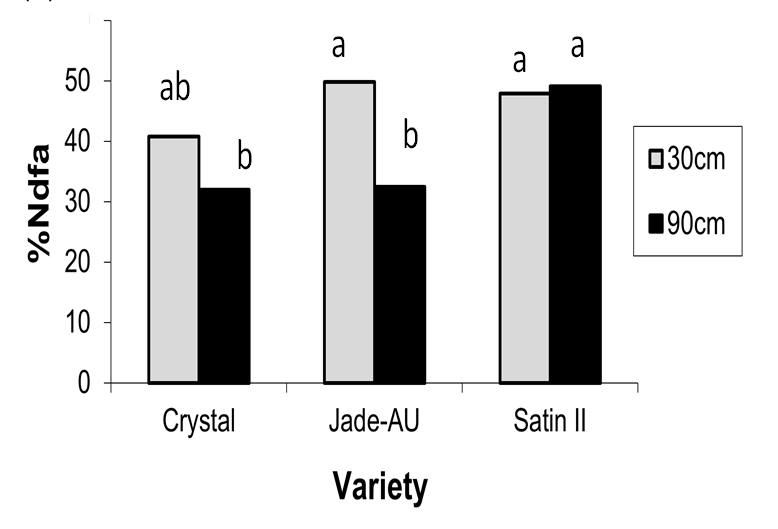
Figure 3. %Ndfa of different varieties at 2 row spacings, Kingaroy 2012/13 (LSD 5% = 9.28).
Time of sowing
Sowing on time to take full advantage of soil water and growing conditions that maximise crop production can make a significant impact on amount of N fixed. The graph below gives an indication of the impact that time of sowing of soybeans can have on N fixation comparing soybeans planted in the middle of the appropriate planting window with late in that window. Figure 4 shows that for two different soybean varieties (NF246-64 and PR443) as much as 150kg/ha less N is fixed due to the shortened growing season. However, increasing the plant population becomes very important for improving the proportion of N that is fixed if time of sowing is delayed (Figure 5).
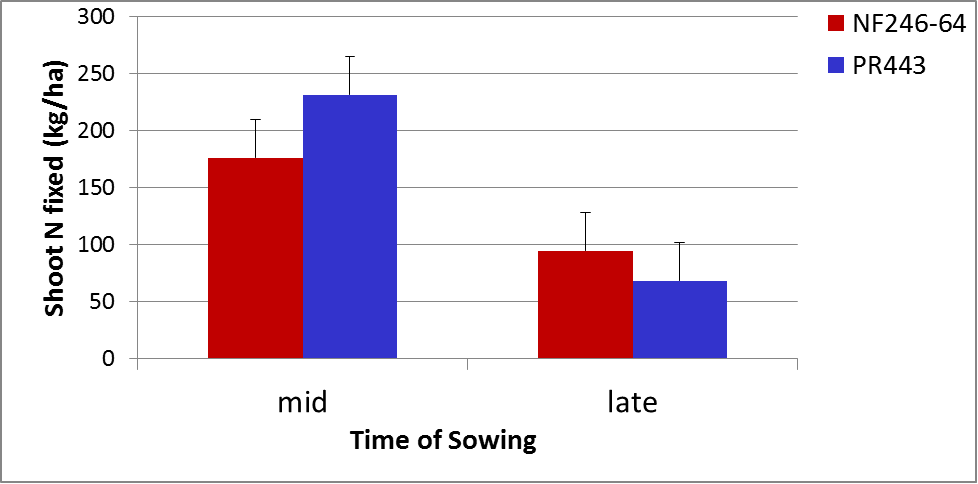
Figure 4. Nitrogen fixation is reduced when soybeans are sown late in the planting window (LSD 5%=33).
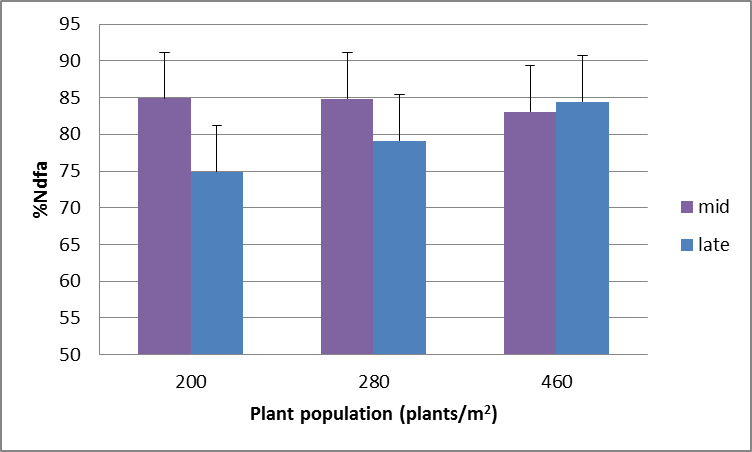
Figure 5. Higher plant populations compensate partially for a later planted soybean crop in terms of N fixation (LSD (5%) = 8.7).
Impact of applied fertilisers
Field trials conducted in Central Queensland in mungbean showed that nodulation was suppressed by applications of urea at rates of 10 or more kg N/ha or Triple Super at 5 or 10 kg P/ha (Figure 6). Also, no yield advantage was gained by the addition of fertiliser N (Figure 7) (Seymour et al. 2010). Pre-plant soil nitrate levels at this site started at about 80kg N/ha in the top 1m and were increased from there with applied fertilisers. There was also a history of mungbeans at this site contributing to the nodulation of the uninoculated treatments. Despite this, there was a significant yield response to inoculation.
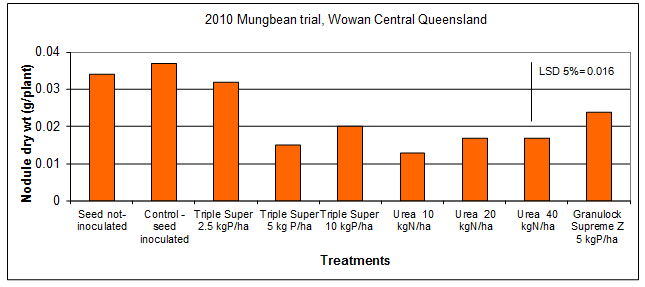
Figure 6. Impact on nodulation of mungbean cv. Crystal from rhizobial inoculation and preplant fertiliser applications.
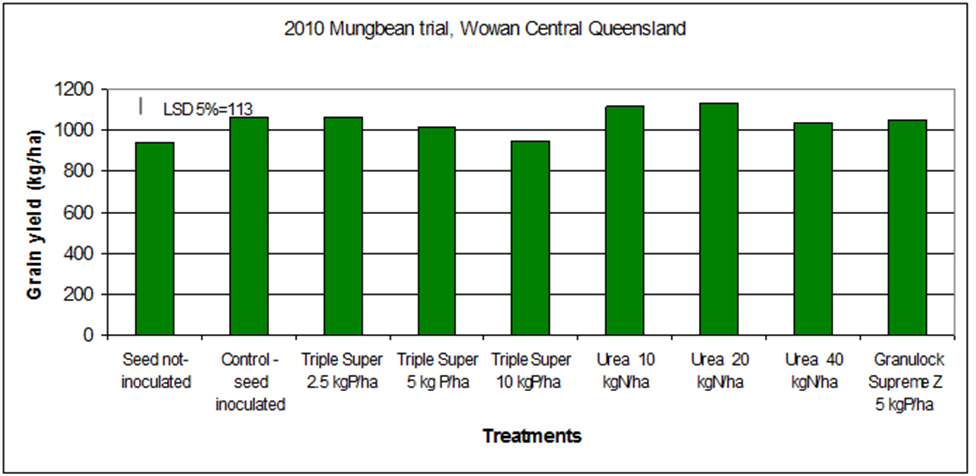
Figure 7. Response of mungbean cv. Crystal to inoculation and preplant fertiliser applications.
Nitrogen fixation in crops is closely linked to biomass production so agronomic practices that improve crop growth all lead to improved N fixation by the crop. Narrower row spacing for pulse crops has particularly shown to improve N fixation compared to wide (1.0m rows) at equivalent plant populations.
Sowing on time to take full advantage of soil water and growing conditions that maximise crop production can make a significant impact on amount of N fixed.
High soil nitrate levels can reduce legume nodulation and N fixation by rhizobia. The addition of N fertiliser does not give any yield advantage in pulses and may reduce the amount of N available for the following crop.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. Many thanks also to all members of the GRDC Pulse Agronomy Initiative in Qld and NSW.
References
Seymour NP, Conway M, Erbacher A, Aisthorpe D and Quinlivan M (2011). Nitrogen fertiliser reduces nodulation of mungbean but gives no yield advantage in Central Queensland. 17th International Congress on Nitrogen Fixation. Fremantle, WA. 27 November -1 December 2011
Doughton JA, Vallis I, Saffigna PG (1993) Nitrogen fixation in Chickpea. I. Influence of Prior Cropping or Fallow, Nitrogen Fertilizer and Tillage. Australian Journal of Agricultural Research 44: 1403 – 13.
Recommended reading
‘Inoculating legumes: A practical guide.’ Ground Cover Direct
Managing legume and fertiliser N for northern grains cropping’ Ground Cover Direct.
Contact details
Nikki SeymourDepartment of Agriculture and Fisheries
Ph: 07 46398837
Fx: 07 46398800
Email: nikki.seymour@daf.qld.gov.au
Was this page helpful?
YOUR FEEDBACK
