Working with natural enemies and living with Russian wheat aphid a new pest of Australian cereal crops
Author: Greg Baker, Kym Perry, Michael Nash (South Australian Research and Development Institute, Waite Campus, Adelaide, SA), Garry McDonald, Julia Severi and Paul Umina (cesar, 293 Royal Parade, Parkville, Victoria). | Date: 18 Aug 2016
Working with nature - the role of natural enemies in agriculture
What are natural enemies, and how many are there?
Not every insect in your crop is a pest! In fact, the vast majority of invertebrates (insects, mites, springtails and other creatures) you will see are benign or beneficial species. `Natural enemies` (NE) is a term used to describe naturally occurring invertebrate species that attack and kill other invertebrates, including pests. As such, they provide free `ecosystem services` (also known as biological control) by suppressing and controlling pest species in your crops. As pest managers typically look for pests, NE usually go unnoticed and consequently their role in agriculture is highly underestimated. In reality, NE make an enormous contribution to pest control and profitable cropping, though as yet there is almost no information on quantifying their benefit in dollar terms in Australian agriculture.
Natural enemies include many species of predators, parasitoids and pathogens. There are at least 123 species of predators recorded in Australian crops; just the tip of the iceberg, but still far more than the number of pest species! Predators kill and consume many insects in their lifetime and tend to feed quite broadly (across a range of prey insect species). Common predators include spiders, lacewings, hoverflies, predatory sucking bugs, lady beetles, ground-dwelling beetles and predatory mites. Parasitoids are wasps or flies that tend to specialise on one (or relatively few) prey species. Female wasps lay eggs inside the body of prey, and the young wasp larva consume the prey from the inside out. Many species of parasitoids commonly attack moth and aphid pests. A range of entomopathogens (insect-attacking pathogens) also occur naturally in the field and when favourable weather conditions prevail they can substantially reduce the infestation levels of some pests (e.g. aphids, diamondback moth, Helicoverpa spp., etc).
NE typically become more visible in crops during the warmer months of spring when insect populations and activity increase generally. In reality, various NE groups actively reduce pest numbers year round in crops. Spiders are extremely diverse and abundant and their role is grossly underestimated. Have you ever seen the sheets of webbing across entire paddocks on cold mornings? Think of the sheer abundance of spiders alone, and how many other insects they must be consuming!
What is the role and impact of natural enemies in reducing pest populations?
Researchers widely agree that NE play a critical role in suppressing pest populations. They reduce both the size and the variability in pest populations, and provide resilience within cropping ecosystems. NE prevent established low pest populations from quickly escalating to high pest populations.
A thorough experimental field study in a horticultural system of Brassica vegetable crops in southern Queensland (Furlong et. al. 2004), demonstrated that the impact of NE on reducing populations of the diamondback moth was significantly higher on farms with management practices designed to conserve NE (marked `IPM` in Figure 1; e.g. scouting and threshold-based spray decisions, overall substantial reduction in insecticide inputs, use of softer chemistry), compared to `conventionally` managed farms (marked `CON` in Figure 1; e.g. no scouting, high insecticide inputs, use of synthetic pyrethroids and organophosphate insecticides) or farms intermediate between the two approaches (marked `INT` in Figure 1).
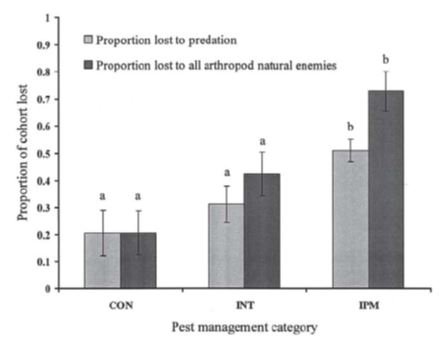
Figure 1: Mortality of diamondback moth cohorts attributed to predators (light bars) or predators and parasitoids (dark bars) on Brassica vegetable farms using management practices designed to conserve natural enemies (`IPM`), compared to conventionally managed farms (`CON`) or farms intermediate between the two approaches Columns of the same color marked by different letters are significantly different (LSD, P < 0.05) (Source: Furlong et al, Journal of Economic Entomology 2004).
However, the details of NE establishment in crops and the impacts of individual NE species or the NE complex on pest populations in the Australian grains agro-ecosystem are largely unknown. To date there has been very little research in this context. Predator-prey interactions are highly complex (often involving multiple species) and measuring the impact of NE requires targeted and carefully-designed ecological studies. Recent advances in molecular tools and ecological techniques are now providing platforms for more reliably measuring NE’s impact in agriculture. This knowledge is critical to effectively manipulate NE in cropping systems and advance the development of non-insecticide-based insect management, and therefore, measuring NE’s impact is an important research area for the Australian grains industry.
Looking after natural enemies and insecticide tools
Insecticides are a valuable pest management tool and, like any resource, need to be looked after. Insecticide applications involve a trade-off between immediate pest control and disruption to NE; creating potential for secondary pest resurgence later in the season. This is particularly true for broad spectrum products, such as synthetic pyrethroids (group 3A) or organophosphates (group 1B), which are highly toxic to many NE.
Insecticide impact on NE in field situations is also poorly understood at present. However, a field study in a soybean crop demonstrated that a pyrethroid insecticide (group 3A) spray greatly reduced the abundance of foliar-dwelling predators in sprayed versus unsprayed plots (each plot 80 x 40m, 0.32ha), and the predators’ population had not recovered during the 20days of post-spray assessments (Figure 2).
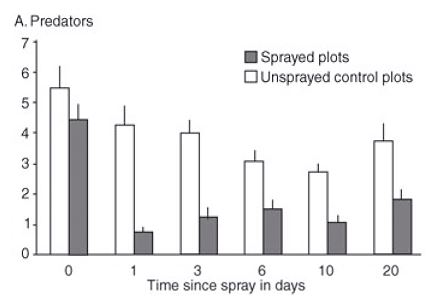
Figure 2: Reduction in predator numbers in soybean field plots sprayed with a synthetic pyrethroid insecticide. Numbers were measured before spray (Time = 0) and 1, 3, 6, 10 and 20 days post-spraying. (Source: modified from MacFadyen and Zalucki, 2012, Insect Science).
In addition to impacts on NE, over-reliance on insecticides increases selection pressure for insecticide resistance in target and non-target pest species (i.e. any insect present in the paddock and exposed to the treatment) as the grains industry is increasingly experiencing with several key pests; including the red-legged earth mite, green peach aphid and diamondback moth.
To work with NE (and preserve available chemistry for the longer term), insecticides should be used strategically, as a last resort in situations where NE control is not able sufficiently reduce pest populations below damaging levels. Prophylactic spraying and indiscriminate use should be avoided.
Strategic insecticide use means:
- Only spraying for pests when needed – i.e. where sampling indicates that pest levels exceed economic threshold (ET) levels. Avoid prophylactic spraying.
- Ensuring correct pest identification and, where required, select an appropriate product (some pests are tolerant to certain groups).
- Using more selective or `softer` products where available (pest-specific products such as pirimicarb and others).
- Rotate chemical mode-of-action groups to help manage resistance.
Be sure to quiz your agronomists and re-sellers to check the availability of different options! (or feel free to contact an author directly for some advice). These principles are highly relevant to the management of the new pest, Russian wheat aphid, which has recently become established in Australia.
Russian wheat aphid - a new pest of Australian cereals
Occurrence and current distribution in Australia
Russian wheat aphid (Diuraphis noxia, RWA) is one of the world's most economically important pests of wheat, barley and other cereal grains. It is native to southern Russia, the Middle East and Central Asia, but since the late 1970s and early 1980s, has rapidly spread to other major grain producing regions in Europe, Africa, North America and South America. Around the world, the distribution of RWA is primarily associated with cereal production regions characterised by warmer, drier climates. It is less prevalent or non-existent in higher rainfall areas.
RWA had not previously been reported in Australia, prior to 19th May 2016 when it was identified in a wheat paddock near Tarlee in the SA Mid North region. Further surveillance has lead to the detection of the species across much of the eastern half of South Australia and western and central Victoria. At the time of publishing this article RWA had not yet been found in the States of Western Australia (WA), New South Wales (NSW), Queensland (Qld) or Tasmania. A map of the known distribution of RWA is being regularly updated on the Plant Health Australia website: (Russian wheat aphid distribution map pdf)
Landholders and agronomists in Victoria and NSW where RWA has not yet been detected are requested to check cereal crops and report any suspect aphids or unusual damage to their State’s Exotic Plant Pest Hotline.
Identification and lifecycle
Wingless adults grow up to about 2mm long and have distinctively short antennae. They are light green in colour and can appear coated with a whitish wax. In addition, there are three other conspicuous features that can help distinguish RWA from other cereal aphids:
- The first is its elongated body. While the bodies of established cereal aphids are often pear or globe-shaped, RWA is longer and more spindle-shaped.
- The second feature is the apparent lack of siphuncles, which are commonly referred to as ‘exhaust pipes’. These structures are notable on most aphids, however they are very difficult to see on RWA with the naked eye.
- The third diagnostic feature is the presence of two caudae or a ‘double tail’ at the rear end of the aphid. This can be seen best when viewing the aphid from a profile perspective.
Adult RWA can be winged (alates) which are also up to 2mm. However unlike wingless RWA, the antennae are body-length, and the body is generally darker in colour.

Figure 3: The Russian wheat aphid is elongated (left) (Source: Helen DeGraaf, SARDI) compared with the globular body of the oat aphid (Rhopalosiphum padi) (top right), and lacks the siphuncles or ‘exhaust pipe’ structure shown on the corn aphid (Rhopalosiphum maidis) (bottom right) (Source: cesar).
In its native range, the annual lifecycle of RWA includes sexual and asexual phases. However, like most other introduced aphid pests in Australia, invasive populations of RWA reproduce asexually with females giving birth to live female offspring.
In autumn, aphids may infest wheat or barley seedlings soon after emergence, usually from wingless aphids walking off nearby senescing hosts. Aphids require actively growing plants for development; populations start to increase from tillering and stem elongation. Aphids regularly move by walking among leaves, tillers and plants, so that the percentage of infested plants increases during the crop cycle. Population growth becomes most rapid from booting onwards. Early in the crop cycle, the vast majority of aphids are wingless. Later in the crop cycle as aphid population density increases, the proportion of winged aphids increases and may reach high levels prior to ripening; at this stage, aphids emigrate in search of alternative summer hosts. Alate RWA are weak flyers, but are thought to travel on wind currents efficiently enough for some aphids to locate isolated host plants.
RWA are able to survive under a wide range of temperatures and may perform better at lower temperatures than the other cereal aphids. The optimum temperature range is considered to be around 18-21°C. RWA does not do well under higher temperatures (>25°C). Under laboratory conditions, generation time ranges from approximately 20 days at 10°C and nine days at 20°C.
Like other aphids, populations of RWA are strongly regulated by environmental conditions. Survival of aphids outside the shelter of leaf rolls is affected by exposure to rainfall, drying winds, and predators and parasitoids. Rainfall washes aphids from upper leaves, and heavy rainfall may cause mortality of up to half of the population. Populations are generally reduced by cold and wet conditions.
Host range and plant damage
The host range of RWA includes more than 140 species of cultivated and wild plants within the family Graminae (grasses). These include wheat, barley, triticale, rye, oats, pasture grasses and wild genera including Poa, Bromus, Hordeum, Lolium, Phalaris and others. Wheat and barley are most susceptible, while triticale, rye and oats are less susceptible. This wide availability of host plants partly explains why RWA had a history of successfully invading new regions. Bromus grasses, particularly perennial species, are important to RWA survival and population build-up. In South Africa, native grasses apparently do not host RWA.
Unlike other cereal aphids that damage plants by removing nutrients, RWA also injects salivary toxins during feeding that cause rapid, systemic phytotoxic effects on plants, resulting in acute plant symptoms and potentially significant yield losses. Even a few aphids can cause plant damage symptoms to appear as early as seven days after infestation. These include:
- Curled, rolled or hollow tube leaves,
- discoloured leaves,
- white and purple streaks on leaves,
- stunted growth or flattened appearance,
- hooked-shaped head growth from awns trapped in curling flag leaf, and;
- bleached heads.

Figure 4: Streaking on wheat leaf (left) (Source: Michael Nash, SARDI) and flattened growth from RWA feeding (right) (Source: Kym Perry, SARDI).
Aphids feed in dense colonies, typically at the base and sheath of younger leaves and within leaves curled by their feeding. Aphids prefer the newest leaves of plants, and are often found on the last two leaves unfurled. At high densities they can be found on any foliar parts.
The salivary toxins injected by RWA during feeding damage plant chloroplasts, resulting in reduced photosynthetic ability, delayed leaf initiation and tillering, and reduced numbers of fertile tillers, shoot and root biomass, grains per ear and grain weight. Yield impacts are determined by the percentage of infested tillers and plants and crop development stage. Heavy infestations during early growth can cause serious damage (under USA conditions). From early booting to soft dough stage, feeding on upper leaves, in the leaf sheath and next to the developing head, can cause direct yield losses. In wheat and barley, damaged leaf tissue does not recover. If aphids are controlled, new growth proceeds normally (new root and shoots are unaffected) and plants may recover unless excessively stressed. After soft dough stage, further impact is minimal.
Management
The GRDC FITE strategy is recommended for RWA aphid control:
Find
Look for aphids and the characteristic plant symptoms on cereal crops and grasses.
Aphids may infest crops during any stage of crop development, from early establishment to maturating flag leaf. Check crops regularly following seedling emergence. RWA are often difficult to find when at low numbers so check for the characteristic and distinctive leaf streaking and rolling. Infestations often begin along crop edges, usually on the windward side or adjacent to infested grasses. RWA also commonly occurs in areas of paddocks where plants are sparse, on sandy rises or adjacent to bare ground. After initial infestation, aphids can rapidly spread across a paddock.
SARDI entomologists have observed weather conditions may affect distribution of aphids on plants. During inclement weather RWA on volunteer cereals (GS5 to GS8) were only found on lower leaves and in their leaf sheaths, but were more broadly distributed over plants during fine weather.
Identify
Positively identify RWA in consultation with a specialist where necessary.
Threshold approach
Consider international thresholds for control, factoring crop growth stage, crop yield potential and potential yield losses.
Chemical control of RWA is effective however decisions on the need for foliar treatments are based on the proportion of seedlings or tillers infested. Threshold guidelines (ET) recommended in the USA vary somewhat between regions, but for early season growth we currently recommend an ET of 20 per cent seedlings infested up to the start of tillering, and 10 per cent plants infested thereafter. Local research will be required to test, and if required, to modify these thresholds for Australian crop conditions.
Due to the cryptic feeding habits of RWA, complete coverage and use of an insecticide with fumigant or systemic activity is required. An APVMA permit (PER82792) has been issued for the use of products containing 500 g/L chlorpyrifos and 500 g/kg pirimicarb to control RWA in cereals. The results of RWA spray trials with a range of products and rates conducted in SA in July will be presented at the Update. To maximize coverage, which is essential for RWA control, we advise using a high water volume (minimum 100 L/ha), a non-ionic surfactant and a nozzle pressure that produces medium-sized droplets (e.g. 2.5-3.0 bar pressure for flat fan nozzles).
Enact
Enact an appropriate management strategy that where possible encourages beneficial insects.
RWA is attacked by a range of natural enemies in other parts of the world, many of which also attack other aphids. Of these, groups that commonly occur in Australia include the minute parasitoid wasps Aphidius colemani, A. ervi, Diaeretiella rapae and generalist predators including ladybird beetles (e.g. Coccinella spp., Hippodamia spp.), lacewings (Chrysopa spp.), damsel bug (Nabis sp.), hoverflies (Syrphus spp.), and also entomopathogenic fungi. We have already observed mummified and fungus diseased RWA. If spraying is warranted, aim to use the softer chemistry to maintain predators and beneficial populations.
We do not advocate the use of prophylactic sprays for managing invading or dispersing RWA. These sprays can create secondary pest outbreaks (such as other cereal aphids or armyworms) by removing beneficial species. Invading (winged) RWA are expected to be more prominent later in the season.
Non-chemical cultural control options include eliminating refuge volunteer cereals and grasses in fallows and other areas during summer and autumn; later planting of winter cereals to delay and reduce early aphid infestation; agronomic practices to promote crop vigour and dense canopy growth which inhibits RWA populations and reduces their impact on the crop.
RWA resistant wheat and barley germplasm is available overseas and some has already been introgressed into certain Australian cereal lines. GRDC and commercial breeding companies are initiating screening and breeding programs to provide industry with resistant/tolerant cultivars in the future.
Acknowledgements
RWA field work in SA is currently funded by the SA Government.
Plant Health Australia RWA national technical group.
References
Furlong, Michael J., et al. "Experimental analysis of the influence of pest management practice on the efficacy of an endemic arthropod natural enemy complex of the diamondback moth." Journal of Economic Entomology 97.6 (2004): 1814-1827.
Macfadyen, Sarina, and Myron P. Zalucki. "Assessing the short‐term impact of an insecticide (Deltamethrin) on predator and herbivore abundance in soybean Glycine max using a replicated small‐plot field experiment." Insect Science 19.1 (2012): 112-120.
Useful resources
Crop Aphids: The back pocket guide. GRDC
GRDC Paddock Practices - Monitor RWA numbers closely over winter
Hughes RD. A synopsis of information on the Russian wheat aphid, Diuraphis noxia (Mordwilko).(Revised edition). CSIRO Australia Division of Entomology Technical Paper. 1996(34).
Pike KS, Allison D. Russian wheat aphid. Biology, damage and management. Pacific Northwest Cooperative Extension Publication. 1991 (PNW371).
Plant Health Australia – Threat Specific Contingency Plan
Contact details
Greg Baker, Michael Nash or Kym PerrySARDI
Waite Building, Waite Rd., Urrbrae, S. AUST.
08 8303 9536
bill.kimber@sa.gov.au
@PestFactsSARDI
Paul Umina, Garry McDonald or Julia Severi
cesar
293 Royal Parade, Parkville, VICTORIA
03 9349 4723
pestfacts@cesaraustralia.com
@PestFactscesar
Was this page helpful?
YOUR FEEDBACK
