Insect pest management research update 2017
Author: Melina Miles, Adam Quade, Richard Lloyd, Paul Grundy and Jamie Hopkinson, DAF Qld | Date: 28 Feb 2017
Take home messages
- Canola is most susceptible to yield and oil loss with infestations of aphids from bolting. This is earlier than previously thought, but still requires at least 10-14 days of infestation to significantly impact on the crop.
- Preliminary thresholds are proposed for helicoverpa in canola.
- Initial observations on helicoverpa feeding behaviour in faba beans suggest a crop loss rate of approx. 2.4g/larva.
Aphid thresholds in canola
Over the past 3 years, DAF entomologist Paul Grundy has been undertaking research to determine the susceptibility of canola to cabbage aphid infestations during flowering and seed set. The initial work, simulating late infestations on the terminals of filling/maturing racemes, showed that canola was compensating for terminal damage and/or the affected terminal pods were not contributing significantly to yield. Recent trials (2016) have shown that cutting stimulates atypical compensation, and that bagging better simulates aphid impact, which is to prevent normal development of racemes (Table 1).
Table 1. Differences in the plant response to simulated aphid damage. Cutting racemes vs constraining growth by bagging racemes. Trial conducted in dryland canola at Allora, SE Qld, 2013.
|
Cutting to simulate aphid damage |
Bagging racemes to simulate aphid damage |
||
|
Treatment |
Yield (t/ha) |
Treatment |
Yield (g/plot) |
|
Control |
2.07 a |
Control (unbagged) |
1293 a |
|
10% of terminals removed |
1.93 a |
1. Raceme covered first flower |
988 b |
|
50% of terminal removed |
1.98 a |
2. Raceme covered first flower +7 days |
989 b |
|
90% of terminal removed |
2.01 a |
3. Raceme covered first flower +14 days |
949 b |
Treatments followed by the same letter are not significantly different (P<0.05).
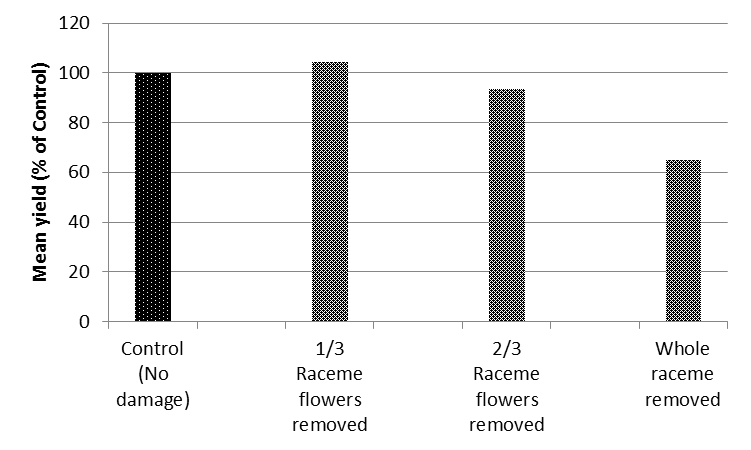
In 2014, trials at 8 locations on the Liverpool Plain produced similar results, with significant yield loss only occurring when the plant was forced to replace all the reproductive structures removed at early flowering (Figure 1).
Figure 1. Aggregate treatment yields across 8 sites near Spring Ridge NSW, 2014, expressed as a percentage of the control.
The conclusion of this research was that the widely used best-bet threshold, of 10-20% of racemes infested during late flowering-pod set, is probably conservative and could be revised up. The compensatory ability of the crop allows time for biological control by aphid parasitoids, lacewings, hoverflies and ladybeetles to take place during the first weeks of flowering without significant crop loss. If natural enemies were ineffective in supressing aphid populations, spraying on increasing level (20-25% of racemes infested) would be unlikely to result in irrecoverable crop damage. Very late infestations of aphids have very limited damage potential as the associated disruption mainly affects flowers/pods that are unlikely to contribute to final yield.
In 2016, replicated trials at Wellcamp near Toowoomba were undertaken with infestations of aphids, rather than simulated damage. This approach provided an opportunity to examine both the impact of aphids on crop growth and oil content. Conditions for crop compensation were excellent with approx. 300 mm of rain between June and September. Plots were infested with aphids prior to bolting to get racemes infested as early as possible. In the early sown trial (sown 9 April, infested 16 July) the infestations did not establish before bolting, but were well established through flowering. Infestation in the later sowing (sown 17 May, infested 10 August) resulted in successful early infestation so flowering racemes were heavily infested from early flowering. Infestations resulted in every plant having some aphids established in each treated plot. Greater impacts on both the yield and oil content were observed in the later sown trial (Figure 2).
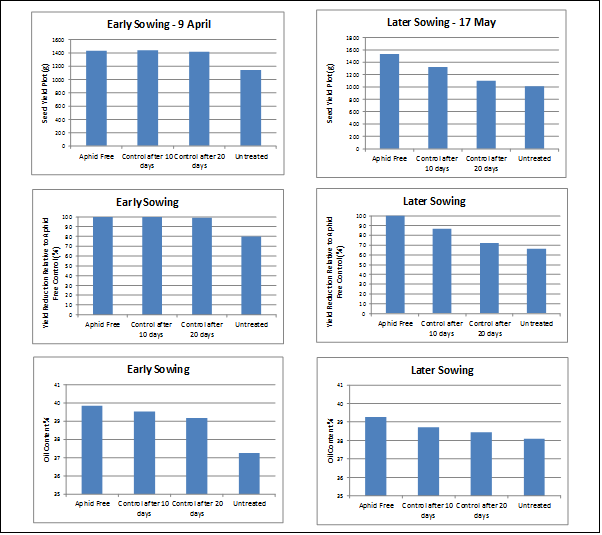
Figure 2. Very early infestation of flowering racemes with cabbage aphid for a 10 day period can reduce both yield and oil content. Result of replicated trials, Wellcamp 2016.
What the 2016 trial results mean for canola aphid management recommendations
The series of trials over the past 3 years have indicated that infestations in early flowering result in greater impacts than later infestations, and this work reinforces this.
The most susceptible period is the 2-4 weeks in which the plant is bolting and starting to flower; this is when crop monitoring for aphids should start – and which is probably earlier than it is done currently. To simplify sampling, assess aphid infestation in terms of % plants infested, rather than % racemes infested.
As significant yield and oil content declines did not occur with infestations of 10-14 days, there is time to assess the rate at which the aphid infestation is growing, and the potential impact of natural enemies and weather on infestations.
At this stage, a threshold of 20% of plants infested is proposed. Further work is required to validate this approach.
Helicoverpa threshold in canola and faba beans
Canola
To date, thresholds available for managing Helicoverpa in canola have been based on ‘best guesses’. In 2015 we conducted a replicated trial to determine the consumption rate of Helicoverpa larvae in canola. A consumption rate is a vital component of an economic threshold, and is an estimate of the yield and crop loss likely to occur. It is calculated from the lifetime consumption of a larva. In other words, it is a reflection of the amount of yield loss that would occur if the larva was not controlled.
In summary, results of the trial are:
(i) The consumption rate of a Helicoverpa larva is estimated to be 2.4 grams of grain per larva.
(ii) On average a larva damaged 10.5 pods and consumed 124 seeds.
(iii) Larvae showed no preference for pod size/maturity
If we use the following equations, we can calculate the potential yield loss and economic thresholds for a range of crop and cost of control values.
Table 2. Calculating potential yield loss and economic thresholds
|
Potential yield loss (t/ha) per larva = D x P x V |
Economic threshold (larvae/m2) = C / (V x D) |
|
Where D = estimated yield loss per larva (t/ha) P = pest density per sampling unit (e.g. per m2) V = crop value ($/t) |
Where C = cost of control ($/ha) V = crop value ($/t) D = estimated yield loss per larva (t/ha) |
These consumption rates are preliminary. Two further trials were conducted to assess consumption rates. This data is not yet fully analysed. It is expected that we will have greater confidence and firm thresholds within 1-2 seasons.
Faba beans
Two replicated cage trials were conducted in 2016 to quantify the damage potential of helicoverpa in faba beans. Whilst this data is not yet fully analysed, the research has provided some insight into the feeding/damage behaviour of helicoverpa in faba beans. Here is a summary of some of the outcomes:
- Each helicoverpa larva damaged 4 seeds. On average, one seed was damaged per pod; and each larva damaged 4 pods.
- The majority of damaged seed (70% in Kingaroy and 100% in Billa Billa) had significant damage to the cotyledon. There was relatively little seed that was only superficially damaged (quality rather than yield impact).
At this stage we can only tentatively propose a crop loss rate for helicoverpa in faba beans, based on broad and, as yet untested, assumptions. For example, if each larva causes significant damage to 4 seed in its lifetime, and we assume each of these is lost to feeding and/or at harvest, then in a crop of Warda (seed weight around 0.6g/seed) each larva would cause a yield loss of 2.4g (or 24 kg/ha for every larva per square metre). We can compare the threshold calculated from this rate of yield loss (ET=C/(VxD), see equation above) to the nominal 2 larvae per sqm commonly used.
ET = 5.0 if using $30 insecticide application and $250/t for grain.
Working with an ET =2, the potential yield loss for grain at $250/ha = $12
Clearly this challenges the perception that even at 2 larvae/sqm significant damage is occurring in faba beans. This is why the quality (partial grain damage, with grain retained at harvest) is important to investigate. Also of importance is effective sampling (method and timing).
In 2017 we will be assessing the potential of damaged seed to be shed, or retained in the harvested grain sample. This will be a key factor in determining the level of impact helicoverpa are having on yield and quality. We also plan to review sampling effectiveness as previous work suggested potential to underestimate larval numbers with current methods.
Late mirid damage in faba beans
In 2016, mirid densities were very low in late winter – early spring; consequently further work on thresholds was not possible this season, but it is a priority for 2017.
To summarise the findings to date:
A replicated trial that caged mirid adults or nymphs on developing and maturing pods was conducted in 2015. The purpose of the trial was to establish conclusively whether mirid feeding caused spotting on the seed, and whether mirids warranted further research as a pest of faba beans.
Pods at two development stages were included in the trial, 1) fully filled, but still green and 2) immature, seed approximately 30% filled. Either 2 adult mirids, or 2 late instar nymphs were confined on a pod for 10 days, and there were control pods on which there were no mirids. At the end of the 10 days the mirids were removed from the ‘cages’ and the cages replaced to protect the pods from being damaged by any other insect or weather. Cages remained on the pods until the pods were mature and dried down (harvestable). During this time cages were checked, and treated where necessary for small nymphs that hatched from eggs laid by the mirids in the trial.

Figure 3. Effect of green mirids caged on faba bean pods of different ages
As shown in the figure above, mirids did consistently cause spotting on the seed coat. Adult and late instar mirids caused similar damage. When filling pods were exposed to mirids, a significant proportion of seed did not develop. This trial result clearly demonstrates the damage potential of mirids in faba beans, both in terms of quality (spotting) and yields (small seed).
This trial was not designed to address the question of threshold. We cannot extrapolate from these data to estimate how many mirids will cause significant seed spotting or screenings. These are areas of research that will be addressed as opportunities arise.
Green peach and canola – overview of the issues
In 2013, the Green peach aphid (GPA) outbreak in canola in southern Australia caused significant concern in the industry. In particular, the transmission of barley yellow dwarf virus (BYDV) and insecticide resistance to multiple modes of action.
Whilst GPA is relatively uncommon in the northern half of the Northern Grains Region, it worth being aware of the impact and potential issues that the use of insecticides may have in promoting GPA populations and/or resistance.
High levels of resistance to carbamates and pyrethroids are now confirmed to be widespread across Australia. Moderate levels of resistance to organophosphates have been observed in many populations, and there is evidence that resistance to neonicotinoids (e.g. imidacloprid) is evolving (Umina et al. 2015)
Resources
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
Support for the field trials was provided by Liz Williams, Trevor Volp and Hugh Brier. The field staff at the Kingaroy research station and the Farming systems trial site at Billa Billa made sure the trial plots were planted and maintained.
Contact details
Dr Melina MilesQueensland Department of Agriculture and Fisheries
203 Tor St, Toowoomba
Ph: 0407113306
Email: melina.miles@daf.qld.gov.au
GRDC Project Code: DAQ00196,
Was this page helpful?
YOUR FEEDBACK