Pulse diseases 2016
Take home messages
- Ascochyta lentis isolates from Mallala and Maitland have caused a susceptible reaction on PBA Hurricane XT lentils in controlled environment tests, as well as on PBA Ace and PBA Bolt. Growers in these regions should manage these cultivars as a potentially higher risk for ascochyta blight.
- Faba bean cultivars have not changed reactions to ascochyta blight since 2015. Screening of isolates of Ascochyta fabae identified three reaction groups — Farah is susceptible to ascochyta blight, PBA Rana and PBA Zahra are partially compromised, while PBA Samira and Nura remain resistant.
- Faba bean growers are encouraged to proactively move toward adoption of newer PBA varieties which show superior seed quality and greater tolerance to agronomic practices than Fiesta, Farah and Nura. This trend continued in 2016 trials as elite NVT lines exhibited less disease to chocolate spot and ascochyta blight, demonstrating the progression of accumulative dual-resistance being developed within the Australian breeding program.
- Chickpea cultivars in the southern region are now all moderately susceptible or susceptible to ascochyta blight, requiring multiple fungicides and a thiram-based seed dressing.
- Bacterial blight (BB) affected some field pea crops following severe frost. Consider avoiding field peas in frost prone paddocks, or, in these paddocks, sow to PBA Oura or PBA Percy which are less susceptible to BB, and remove stubble before sowing to reduce the frost risk.
- Cucumber mosaic virus (CMV) and bean yellow mosaic virus (BYMV) infected pulse crops in the upper Yorke Peninsula, Mid and Upper North. These are seed borne viruses, so growers with affected crops should source new seed, or have seed tested for virus infection.
- The presence of sclerotinia in canola and pulse crops during 2016 will mean an increased risk of infection for pulse and oilseed crops from soil borne sclerotes for up to five years, particularly in wet seasons. Cereal rotations in infected paddocks will reduce the risk. Sclerotes in grain samples are not harmful to stock or humans.
Ascochyta blight in pulse crops
Lentils
Ascochyta lentis isolates collected in 2016
Leaf lesions of ascochyta blight (AB) were observed on PBA Hurricane XT lentils in the Maitland and Mallala regions in 2016. Lesions were collected and eight isolates were tested against a number of cultivars in controlled environment conditions at SARDI (Table 1). A susceptible reaction was observed on PBA Hurricane XT lentils in these tests and disease reactions were also observed on PBA Ace and PBA Bolt, although field reactions have not been confirmed on the latter two cultivars. In 2017, growers in these regions should manage these cultivars as a potentially higher risk for AB, in particular thiram-based seed dressing should be used and growers should be prepared for a fungicide spray during podding. Crops should also be monitored closely in case an additional spray is required during the season to control AB, especially in a wet season. PBA Jumbo2 and Boomer exhibited a resistant reaction to these eight isolates.
Table 1. Reaction of lentil cultivars when inoculated with Ascochyta lentis isolates collected from PBA Hurricane XT in 2016.
| Isolate collection site | Host Varieties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cumra (susceptible check) | PBA Hurricane XT | PBA Ace | PBA Bolt | PBA Giant | PBA Herald XT | PBA Blitz | Boomer | PBA Jumbo2 | |
| Maitland | 2.5 | 10.0 | 4.5 | 0.8 | 1.7 | 3.8 | 2.7 | 1.9 | 1.0 |
| Sunnyvale | 6.3 | 5.2 | 4.8 | 2.9 | 1.0 | 2.3 | 1.7 | 1.5 | 1.0 |
| Mallala | 6.3 | 13.1 | 4.8 | 2.7 | 1.9 | 4.0 | 4.0 | 3.1 | 1.3 |
| Maitland | 7.9 | 13.3 | 7.9 | 3.3 | 1.7 | 6.9 | 5.0 | 2.9 | 0.8 |
| Maitland | 14.0 | 5.6 | 4.8 | 4.4 | 10.0 | 2.3 | 2.7 | 1.7 | 0.2 |
| Maitland | 15.8 | 10.2 | 6.5 | 6.7 | 8.5 | 4.4 | 5.0 | 3.1 | 2.1 |
| Arthurton | 16.0 | 5.6 | 10.8 | 7.1 | 5.8 | 5.8 | 2.7 | 1.9 | 0.2 |
| Mallala | 18.3 | 10.0 | 6.7 | 12.9 | 5.4 | 1.9 | 2.1 | 4.0 | 0.6 |
Average %disease of whole plants when inoculated in controlled screening with A. lentis isolates collected from PBA Hurricane XT in 2016.
lsd=4.2
0-4.2 Resistant (R)
4.3-8.4 Moderately Resistant (MR)
8.5-12.6 Moderately Susceptible (MS)
>12.7 Susceptible (S)
Ascochyta lentis isolates collected in 2015
In addition to the above testing, 40 isolates of A. lentis collected in 2015 from lentil field trials and commercial paddocks were tested on a differential host set that included PBA Hurricane XT and Nipper (Table 2). On Nipper, the majority of the isolates caused a moderately susceptible or susceptible reaction, and only two caused a moderately resistant reaction, confirming the widespread loss of foliar AB resistance in Nipper. Nipper requires a fungicide spray during podding to prevent pod and seed infection and crops should also be monitored closely in case an additional spray is required during the season. In contrast to the findings in Table 1 above, none of the 2015 isolates caused a susceptible or moderately susceptible reaction on PBA Hurricane XT, indicating that the susceptible reactions noted above on PBA Hurricane XT are a very recent change in the population.
Table 2. Forty Ascochyta lentis isolates collected in 2015 were inoculated onto a lentil host differential set in controlled conditions. Entries in the Table are the number of isolates per category.
| Test reaction | Cumra (susceptible check) | Nipper | PBA Hurricane XT | ILL7537 (resistant line) | Indianhead (resistant line) |
|---|---|---|---|---|---|
| R | 0 | 0 | 29 | 36 | 38 |
| MR | 0 | 2 | 11 | 4 | 2 |
| MRMS | 2 | 19 | 0 | 0 | 0 |
| MS | 10 | 14 | 0 | 0 | 0 |
| S | 28 | 4 | 0 | 0 | 0 |
Faba beans
Ascochyta fabae isolates collected in 2015
Forty isolates of A. fabae collected in 2015 from faba bean field trials and commercial paddocks were tested on a differential host set that included the Australian commercial cultivars. Faba bean cultivars have not changed reactions to AB since 2015 (Table 3) and screening of the isolates identified three reaction groups— Farah is moderately susceptible to AB in the Lower to Upper North of SA, PBA Rana and PBA Zahra are partially compromised, while PBA Samira and Nura remain resistant. Testing of a smaller set of isolates collected in 2016 has confirmed that these new pathotypes are also starting to become established in the Yorke Peninsula and South East growing regions. A three spray fungicide strategy is now required to control AB in Farah, while podding sprays should be planned for PBA Rana and PBA Zahra to prevent pod and seed infection.
Table 3. Forty Ascochyta fabae isolates collected in 2015 were inoculated onto a faba bean host differential set in shadehouse conditions. Entries in the Table are the number of isolates per category.
| Test reaction | Icarus | Farah AR | PBA Zahra | PBA Rana | PBA Simira | Nura AR |
|---|---|---|---|---|---|---|
| R | 0 | 3 | 3 | 4 | 17 | 38 |
| MR | 0 | 1 | 7 | 7 | 17 | 3 |
| MRMS | 0 | 5 | 26 | 16 | 5 | 0 |
| MS | 1 | 25 | 4 | 14 | 2 | 0 |
| S | 40 | 7 | 1 | 0 | 0 | 0 |
Chickpeas
A virulence change in the AB pathogen of chickpeas in southern Australia now means that all current varieties are rated as either susceptible or moderately susceptible to AB infection (Table 4). This follows observations of severe AB on previously resistant chickpea varieties in 2015 and 2016 across South Australia (SA) and Victoria (VIC). Chickpea growers now need to carefully consider their risk to AB infection along with the ability to effectively control the disease prior to choosing to grow this crop in southern Australia. This will be the case in both high and low rainfall regions as severe disease outbreaks can still occur in the latter in all current variety options during wet seasons such as 2016. All chickpea crops will need to be regularly monitored for AB infection.
Table 4. Ascochyta blight categories of chickpea cultivars and disease scores from trials in SA and VIC in 2016 show all cultivars are now either moderately susceptible or susceptible to this disease.
| Variety | Ascochyta blight foliage disease scores 2016 | ||
|---|---|---|---|
| Rating for 2017 | Curyo Vic 1-9 disease score | Kingsford SA 1-9 disease score | |
| Desi type | |||
| Ambar | MS | 5.8 | 6.3 |
| Genesis™ 509 | MS | 6.0 | |
| Howzat | S | 8.0 | 9.0 |
| Neelam | MS | 6.3 | 5.7 |
| PBA HatTrick | S | 7.7 | |
| PBA Maiden | S | 7.0 | 7.0 |
| PBA Slasher | MS | 7.3 | 5.3 |
| PBA Striker | S | 7.5 | 7.3 |
| Sonali | S | 8.8 | |
| Kabuli type | |||
| Almaz | MS | 6.3 | 6.3 |
| Genesis™ 079 | S | 7.0 | 5.0 |
| Genesis™ 090 | MS | 6.7 | 3.7 |
| Genesis™ 114 | S | ||
| Genesis™ Kalkee | MS | 5.5 | 4.7 |
| PBA Monarch | S | 7.5 | 8.0 |
| LSD (P=0.05) | 1.7 | 1.5 | |
- Moderately susceptible varieties will generally require three to four strategic fungicide sprays ahead of rain events offering 2-3 weeks protection, starting at 6-8 weeks post sowing.
- Susceptible varieties will require regular fungicide sprays every 2-3 weeks throughout the growing season in front of rainfall events.
- As the pods of all commercial varieties are susceptible to AB, they will also require fungicide sprays during pod setting ahead of rain fronts to protect the pods from seed staining and seed abortion.
- It is imperative that all chickpea seed is treated with a thiram based fungicide to prevent seed transmission of AB onto the emerging seedlings in 2017.
- The disease will also survive on stubble and organic matter for a number of years, so growers must observe a minimum of a three year rotation between chickpeas in the same paddock, and avoid planting adjacent to last year’s chickpea stubble.
Botrytis diseases in pulse crops
Botrytis grey mould (BGM) was reported in early sown lentils sown into narrow row which produced heavy canopies thereby promoting this disease. All lentil crops, irrespective of BGM status, in SA should be treated with a canopy closure fungicide spray and follow up sprays are determined by seasonal conditions. The majority of crops had minimal damage from BGM in 2016. Chocolate spot was reported in many faba bean crops, especially those sown early which produced heavy canopies, thereby promoting this disease. All commercial varieties of faba bean, irrespective of differences in chocolate spot susceptibility, should be treated with a pre-canopy closure fungicide spray and follow up sprays are determined by seasonal conditions. The majority of crops had multiple applications of fungicide, but older varieties (Fiesta and Farah) were reported to have higher disease levels than newer releases (PBA Samira).Commercially available varieties and advanced faba bean lines in the NVT program were assessed for disease at the Freeling site in the Lower North in 2016. The extended wet season allowed data of AB in August and chocolate spot in October to be gathered at the site. Results presented in Figure 1 show that new advanced NVT lines (triangles) clustered together with a significantly lower level of both diseases compared to older varieties which clustered together with higher levels of disease. Current PBA releases clustered between these groups. This demonstrates the progression of accumulative dual-resistance being developed within the breeding program.
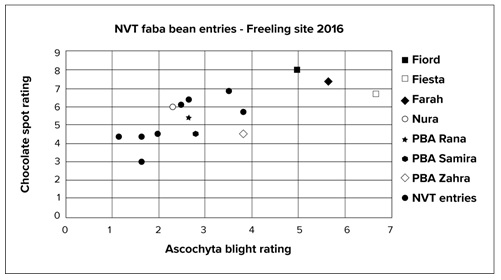
Figure 1. Ascochyta blight scores plotted against chocolate spot scores from Freeling in 2016 for current commercial faba bean varieties and nine NVT faba bean entries.
Field mould in faba beans
A three-year SAGIT funded project was concluded this year examining agronomic factors that may affect the incidence of field mould in faba bean seed. The project provided valuable information and advice on agronomic practices that may mitigate the incidence of field mould, or other factors affecting seed quality and subsequent market access. There were several key conclusions that could be drawn from results of field trials and crop surveys conducted in 2013, 2014 and 2015:
- Variety has a significant effect on seed quality and the impact of in-crop practices. Fiesta (and Farah when included in trials) was consistently shown to have poorer seed quality, and greater detrimental response from in-crop practices that may reduce seed quality, than newer varieties such as PBA Rana, PBA Samira and PBA Zahra.
- The incidence and severity of pod wall residue, which could be confused in some instances as field mould in its appearance, was found to be a heritable characteristic. Older varieties such as Nura were found to be most susceptible and the breeding program is, therefore, selecting against this trait commencing the F3 generation.
- Mechanical damage (wheel-tracks from in-crop traffic) could affect seed quality by reducing grain weight and increase staining on seed within wheel-tracks compared to the standing crop. However, this effect was significantly exacerbated in Fiesta and Nura compared to PBA Rana. Furthermore, this effect varied between seasons and surveyed crops. One likely explanation is that ‘flattened plants’ were actively ‘picked-up’ during harvest in field trials or surveyed plants to examine seed, whereas this may not occur in large scale commercial applications.
- The practice of windrowing in itself had little effect on seed quality. However, significant differences in colour, grain weight and uniformity observed in some trials were associated with variety (PBA Rana being unaffected, whereas detrimental effects were often shown in Fiesta and Nura). In addition, while the timing of windrowing (early, mid and late) had little effect on seed quality, late windrow treatment was combined with a late harvest treatment (mimicking the effect of leaving windrow for a longer period before pickup), did reduce seed quality.
- Crop topping timing can have a significant effect on seed quality. Early implementation of crop topping prior to onset of seed maturation can increase seed shrivelling and weather staining and reduce grain weight, as well as often diminish uniform colour and size.
- The inclusion of a prophylactic fungicide, for example, chlorothalonil, included at the timing of crop topping often negated the negative impact observed on seed quality by early implementation of crop topping prior to onset of seed maturation. Whilst optimal timing of crop topping is the preferred recommendation, this effect was observed in cv. Fiesta within the Mid-North trials in 2014 and 2015, whereby fungicide treatments significantly reduced seed staining and the negative effects on grain weight in early crop topping treatments compared to those without a fungicide included at that timing.
Sclerotinia in pulses and canola
In canola, fungicide applications at 20-30% bloom (15-20 open flowers off the main stem) can prevent main stem infections. Note that bloom is defined as the number of flowers per plant, not the number of flowering plants within the crop. The fungicides give approximately three weeks protection. Current registered fungicides for sclerotinia in canola include Prosaro® and various others containing iprodione and procymidone. There are no fungicides registered for sclerotinia control in pulses, but applications for the control of BGM and chocolate spot should assist with reducing sclerotinia.
Sclerotes in the harvested grain can cause rejection of the grain at silos and suspect grain should be graded to remove the sclerotes. There is no evidence that sclerotes are harmful to stock or human health.
Bacterial blight in field peas
Severe bacterial blight (BB) in field peas was reported in the Mid and Upper North and Upper Eyre Peninsula, no doubt enhanced by the frost, which provides a means for the bacteria to infect plant cells. Classic BB presents as fan shaped lesions at the base of the leaf where it is attached to the stem, and spreads up and down the stem. Infection can start in one patch in a paddock and then spread over a wide area. Unfortunately, there is nothing that can be done to protect the crop or help it recover. The main recommendation is to stay out of the crop to prevent the disease being spread further on tyres or on boots. Infected crops should be the last pea crops to be harvested. This is to prevent the trash from infecting pea grain of non-infected crops. No grain should be kept from infected crops as there is a high chance of seed infection. If a crop has only a small area infected, then it is possible to harvest a clean area for seed.
When planning to sow field pea crops, growers need to consider that if a paddock is frost prone — it is best to sow field peas into a fallow rather than retaining the stubble. This is because the stubble increases the risk of frost, which in turn increases the risk of BB. The preferred field pea varieties to grow in frost prone areas are PBA Oura or PBA Percy, which are less susceptible to BB than other varieties.
Aphids and virus infection
Cucumber mosaic virus (CMV) and bean yellow mosaic virus (BYMV) severely infected some pulse crops in the northern Yorke Peninsula and Mid and Upper North regions during spring flights of aphids. Symptoms of yellowing and/or stunted plants were seen in chickpeas and lentils, while symptoms in lupins, that is, blackened tillers and wilting plants, were mostly caused by BYMV. It is generally too late to spray for aphids once virus symptoms appear, especially for the non-persistently transmitted viruses such as CMV and BYMV. Seed should not be kept from infected crops as some viruses are seed transmitted, however if retaining seed from infected or neighbouring crops, the grain should be tested for virus infection.
Useful resources
Bacterial blight
Strategies to minimise bacterial blight in field peas
Sclerotinia
Sclerotinia Stem Rot in Canola Fact Sheet
NSW DPI Winter Crop Variety Sowing Guide
Clearing the confusion about sclerotes found in chickpea and lentil crops during 2016 harvest
Fungicides in pulses
2016 Fungicide guides for chickpea, faba bean and lentil crops
Plant and Seed testing for viruses
DDLS Seed testing and certification (Perth)
Crop Health Services, AgriBio Centre, Bundoora, Victoria (Melbourne)
TASAG ELISA Testing Services (Hobart)
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
The authors would also like to thank Dr Kurt Lindbeck, NSW DPI Wagga Wagga for his input with research project and paper.
Contact details
Sara Blake
GPO Box 397, Adelaide
08 83039383
sara.blake@sa.gov.au
Jenny Davidson
GPO Box 397, Adelaide
08 83039389
jenny.davidson@sa.gov.au
Rohan Kimber
GPO Box 397, Adelaide
08 83039380
rohan.kimber@sa.gov.au
Sarah Day
SARDI Clare
08 88426264
sarah.day@sa.gov.au
Larn McMurray
SARDI Clare
08 88426265
larn.mcmurray@sa.gov.au
Was this page helpful?
YOUR FEEDBACK
