Topsoil pH stratification impacts on pulse production in South East Australia
Author: Helen Burns, Mark Norton and Peter Tyndall (NSW Department of Primary Industries, Wagga Wagga Agricultural Institute, Wagga Wagga). | Date: 14 Feb 2017
Take home messages
- Acidic layers below 5cm adversely affect root growth and architecture, nodulation, plant vigour and N2 fixation potential of acid-sensitive pulses.
- Moderately (pHCa 4.6 – 5.0) and severely (pHCa <4.5) acidic layers in the 5-20cm soil profile are not detected using soil samples collected at standard depths of 0-10cm and 10-20cm. Finer sampling at 5cm intervals is recommended to detect pH stratification.
- The current standard industry practice of spreading lime with no incorporation and sowing with knife point press wheels or disc seeders confines the lime effect to the surface layers.
- If severe pH stratification is detected, incorporate lime to a depth of 10cm with a full cultivation operation at least 6-12 months before sowing acid-sensitive species.
- Use appropriate lime rates to maintain pHCa > 5.5 in the entire top 10 cm layer.
- The effect of pH stratification on more acid-tolerant species, including canola, lucerne and cereals should be monitored.
Background
While faba bean, lentil and chickpea are generally acknowledged as being sensitive to soil acidity, they are successfully grown on slightly acidic soils (pHCa >5.0 - 6.0) in the high and medium rainfall zones of south eastern Australia, albeit with somewhat inconsistent yields. This paper focuses on the preliminary findings of a NSW Department of Primary Industries project, supported by GRDC, aimed at identifying factors limiting the production and N2 fixation of pulse crops grown on acidic soils in the grain production regions of south eastern Australia with a long-term average annual rainfall greater than 500mm.
In NSW, these regions are dominated by acidic soils (0-10cm; pHCa < 6.0). Acid-tolerant lupin species make up 49% of the southern NSW pulse area (Richards et al 2016), but adoption of more acid-sensitive species, such as faba bean, lentil and chickpea is limited by inconsistency of yield and perceived high production risk. While field pea show intermediate sensitivity to soil acidity, its susceptibility to disease and frost, limits its potential in the high rainfall zone (HRZ) (E Armstrong pers. comm.).
Very little detailed agronomic research has been reported that examined the response of pulses to soil acidity. Guidelines for tolerance of pulses and rhizobia to soil acidity are inconsistent and vague, for example:- Anon (2015) proposes the ideal pHCa for faba bean is 6.0 – 8.0, but also indicates that pHCa > 5.2 is suitable.
- While Drew et al (2012) indicate the optimal pHCa range for Rhizobium spp. used for faba bean, lentils, chickpea, vetch and field pea is > 6.0, although these rhizobia species are sensitive to pHCa < 5.0.
Numerous studies report the presence of acidic layers at 5-15cm (to 20cm in sandy soils) in both agricultural and non-agricultural systems (for example, Conyers and Scott 1989; Paul et al 2003). Our research investigates the effect of soil acidity below 5cm depth on nodulation and plant growth. Pulse crop and soil data collected from commercial paddocks in 2015 and 2016 have shown the detrimental effect of moderately to severely acidic layers below 5cm on root growth, nodulation and crop vigour. Results indicate that even at sites where lime application has increased soil pH sufficiently to enable acceptable production from canola and lucerne crops, pH stratification and moderately (pHCa 4.6 – 5.0) and severely (pHCa <4.5) acidic layers below 5cm depth may still be present and limit pulse crop growth, production and N fixation.
Our findings are likely to be relevant to acid-sensitive pulses grown on acid soils across all rainfall zones. Furthermore, the severity of the acidity below 5cm depth at a number of sites is sufficient to be affecting the productivity of the main crop and pasture species, including cereals, canola and lucerne.
Method
In 2015 and 2016, a total of 39 commercial legume crops were monitored in NSW, Victoria, SA and Tasmania (Figure 1). The 2015 sites were chosen to achieve geographical spread across acid soil regions of the target zones and included 12 paddocks of faba bean, two of narrow-leaf lupin and one of field pea. Sodosols were the dominant soil type at these sites. In 2016 an additional five growers were engaged in order to investigate a broader range of pulses and soil types — Sodosols, Chromosol and Rudosols (alluvial). Sites monitored in 2016 were sown to faba bean (14), narrow-leaf lupin (2), chickpea (3) and lentil (3).
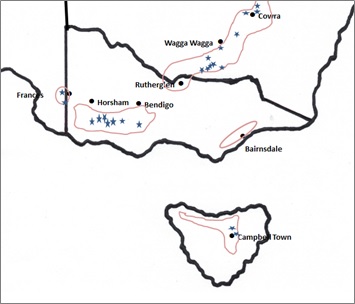
Figure 1. The acidic soil region of the high rainfall cropping zone of south eastern Australia showing the location of paddocks monitored in 2015 and 2016.
A uniform, one hectare area of crop was selected at each site. Soils were sampled at depths of 0-10cm and 10-20cm, with pH measured using the calcium chloride method through Nutrient Advantage Laboratories.
Crop plants were assessed 2-3 months post-emergence for effectiveness of nodulation each year. Plants with intact root systems were collected at random from the designated areas and scored for nodulation using the Columbia protocol (Anon 1991). Scores were allocated for:
- Plant growth and vigour.
- Nodule number.
- Nodule position.
- Nodule colour.
- Nodule appearance.
In 2015, crops with low nodulation scores (less than 18) were investigated further. Root growth was assessed in situ and soil samples were collected at 2.5cm intervals to a depth of 15cm and tested for pH using a Manutec® Soil pH Test Kit. In 2016 root growth was assessed in situ and soil cores were collected from all sites and divided into increments of 2.5cm to a depth of 10cm, and 5cm increments from 10-20cm. Soil pH was measured in the NSW DPI Wagga Wagga laboratory.
Results and discussion
Faba bean was the most commonly grown pulse species in this study, enabling identification of common constraints across NSW, SA and Vic environments, which are also likely to be relevant to other acid-sensitive legume species. It was found that nodulation of faba bean was adversely affected by low soil pH in 2015 and 2016.
Soil acidity and nodulation
Analysis of the nodulation scores for faba bean crops and pHCa of 0–10cm soil samples from the monitored paddocks (Figure 2) showed a strong correlation (r2=0.89) between soil acidity and nodulation scores (0 = nil nodules present, to a maximum of 25 = all plants with effective nodules). The form of inoculant used (peat slurry, freeze dried or granular) did not have a significant effect on nodulation score.
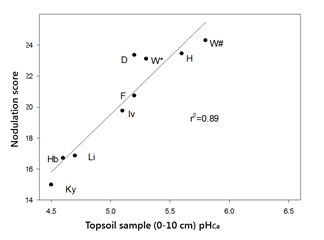
Figure 2. The effect of topsoil pH (0-10cm) on nodulation of faba bean across the south eastern Australian high rainfall zone in 2015. Sites of sampling include Kybybolite, S.A. (Ky), Holbrook, NSW (Hb), Lismore, Vic (Li), Inverleigh, Vic (Iv), Frances, SA (F), Darlington, Vic (D), Willaura, Vic (W) and Henty, NSW (H). W* = after wheat, W# = after canola.
The monitored crops fell into two distinct categories:- Vigorous, well nodulated crops.
- Those with a nodulation score below 18, which included extremely variable crops that showed symptoms of nitrogen deficiency within two to three months of emergence, particularly at the Holbrook (Hb) and Kybybolite (Ky) sites, which recorded nodulation scores of 17 and 15, respectively.
All 2015 sites, with the exception of Kybybolite, had received applications of lime within the last five years. Lime had been applied at the Holbrook site in 2010 and again in 2015 at a rate of 2t/ha. A Speedtiller® was used in 2015 to mix the lime into the surface layers.
The association between nodulation score and soil pHCa was also evident in crops assessed in 2016. This paper will focus on sites J1 and J2 with a faba bean crop growing on red chromosol soil at Junee, (shown as the stars labelled J1 and J2) in Figure 3; and a chickpea crop growing on brown chromosol soil at Woodstock, east of Cowra (shown as diamonds labelled W1 and W2). The nodulation scores were 20.5 and 16.6 for the Junee sites and 20.7 and 15.5 for the Woodstock sites.
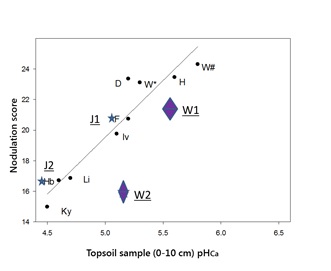
Figure 3. The effect of topsoil pH (0-10cm) on nodulation of faba bean across the SE Australian high rainfall zone in 2015 with 4 sites added in 2016. Stars marked J1 and J2 represent sites at Junee (NSW) growing faba bean, with W1 and W2 Woodstock (NSW) sites growing chickpea.
Sites J1 and J2 are within the same paddock at Junee that had received a blanket lime application in 2011 at a rate of 1.13t/ha, which was not incorporated.
Sites W1 and W2 are also within the same paddock at Woodstock. Lime had been applied at a rate of 2.5t/ha in 2008 and incorporated using a disc plough. In 2016 the grower established test strips with (W1) and without prilled lime (W2). Using knife points, he drilled the lime to a depth of about 10cm, through the fertiliser box of his combine, which was set to deliver prilled lime at a rate of 290kg/ha. GPS guidance allowed him to then sow the chickpea directly over the prilled lime row with a second run.
Soil pH stratification
The only observations and soil test results that will be discussed in detail in this paper are those relating to the 2015 faba bean crops at Holbrook, NSW (Hb), Kybybolite, SA (Ky), and the 2016 sites at Junee, NSW (J1 and J2) and the chickpea crop at Woodstock, NSW (W1 and W2, Figure 2). The responses of crops to soil pH at these sites are consistent with the observations made on all monitored crops growing in acidic soils, across the range of soil type and seasonal conditions experienced in 2015 and 2016.
As shown in Tables 1 and 2, composite soil samples taken at depths of 0-10cm and 10-20cm, which are traditionally used by growers and advisers to guide decisions on acid soil management, failed to detect significant variation in soil pH down the profile at the Holbrook, Junee or Woodstock sites.
The severe pH stratification identified by testing at 2.5cm layers demonstrated that lime incorporation was ineffective under the no-till systems adopted by the majority of participating growers. The lime was concentrated in the surface layers (0-5cm) with little movement of the lime effect below these layers. Clearly, under no-till systems, lime topdressing with no incorporation is ineffective in neutralising acidity below about 5cm depth. Failure to incorporate lime, limits lime reactivity and potential crop response, which is an opportunity cost to growers.
Table 1. The pHCa measurements of 0-10cm and 10-20cm depths underestimate the pH stratification in the soil profile at the Holbrook, Kybybolite and Junee sites, compared with tests from finer sampling increments. Soil conditions at each site are reflected in the appearance of faba bean plants.
| Soil depth (cm) | Holbrook site - 2015 | Kybybolite site - 2015 | Junee 1 site - 2016 | Junee 2 site - 2016 | ||||
|---|---|---|---|---|---|---|---|---|
| Soil pHCa | Soil pHCa | Soil pHCa | Soil pHCa | |||||
| Nodulation score - 17 | Nodulation score - 15 | Nodulation score - 20.6 | Nodulation score - 16.6 | |||||
| Composite sample | Sub samples * | Composite sample | Sub samples * | Composite sample | Sub samples * | Composite sample | Sub samples * | |
| 0-2.5 | 4.6 (Grower's paddock soil test - 5.2) | 6.5 | 4.5 | 4.2 | 5.19 | 5.53 | 4.43 | 4.86 |
| 2.5-5.0 | 5.6 | Not tested | 5.44 | 4.55 | ||||
| 5.0-7.5 | 4.4 | Not tested | 5.15 | 4.22 | ||||
| 7.5-10.0 | 4.2 | 4.0 | 4.63 | 4.07 | ||||
| 10.0-15.0 | 4.1 | 4.1 | 5.7 | Not tested | 4.81 | 4.60 | 4.37 | 4.19 |
| 15.0-20.0 | 4.1 | Not tested | 5.02 | 4.55 | ||||
| Plant appearance | Plants yellow, stunted. Roots were concentrated in top 6 cm; stunted thickened and distorted, typical of Al toxicity. Roots of <10% of plants extend below 10 cm. Dark coloration of roots due to disease – likely due to multiple stresses. | Very poorly nodulated, stunted, yellow plants. Root growth confined to top 10 cm; roots are stunted, thickened and distorted despite soil testing <2% aluminium. Minimal root hair development. The site had no history of lime. |
Healthy, vigorous plants with good nodulation, approx. 35 cm tall at first node growth stage (Sept.). Health and density of roots, including finer root hairs superior to plants from J2 site. Roots restricted to top 10 cm. |
Most plants were yellow and less vigorous, less root hair development than plants from J1. Height 15- 25 cm. Root growth concentrated in the top 4 cm, with minimal root growth below 4 cm. Root disease evident on most plants. |
||||
*Sub samples were not collected from same location as composite samples. pHCa for Hb and Ky sub samples estimated using Manutec® Soil pH Test Kit; pHwater was converted pHCa using the relationship: pHCa =1.012pHW – 0.768 (Conyers and Davey 1988).
The effect of acidic layers on root development and nodulation of faba bean
Despite the Holbrook site receiving 4t/ha of lime since 2010, incorporation with a Speedtiller® was ineffective in mixing the lime below 5cm. Finer soil sampling of the topsoil indicated that at a sowing depth of about 6cm the faba bean seedlings and rhizobia were exposed to a hostile environment (pHCa <4.4), two pH units more acidic than the surface soil (pHCa 6.5). Nodulation was poor and the crop was showing symptoms of severe nitrogen (N) deficiency within three months of emergence.
Root growth was restricted to the surface 6cm and did not penetrate into the severely acidic soil below 5cm.
The results and observations from the Kybybolite site are included to highlight the impact of low pH on faba bean growth and nodulation. Root hair development was poor and plant roots were stunted, thickened and distorted, all symptoms typical of aluminium toxicity. However with exchangeable aluminium levels of less than 2% it is likely that low pH at this site is primarily responsible for the restricted root growth, reduced rhizobial activity and inefficiency of the nodulation process as reported by Cregan and Scott (1998).
The Junee sites were from lower slope (J1) and mid slope (J2) areas within the same paddock. The soil tests from J1 indicate slight acidity (pHCa > 5.0) from 0- 7.5cm, tending toward moderately acid (pHCa > 4.6) from 7.5-15.0cm. Plant roots from this area appeared healthy and were well nodulated (nodulation score of 20.6) but root growth was restricted to the top 10cm. The moderately acidic layers at 7.5 to 15cm (pHCa approx. 4.6) may be responsible for the shallow rooting depth, but the intermittent waterlogging experienced during July to September of 2016 was likely to have compounded the stress caused by the acidic layers.
The J2 soil tests indicated moderate acidity in the surface 2.5cm (pHCa 4.86), tending to severe acidity from 5.0-15.0cm, with pHCa ranging from 4.55 at 2.5cm, to as low as 4.07 at 7.5- 10cm. In contrast with plants from the J1 site, J2 plants were stunted and showed symptoms of severe N deficiency two months after sowing. Root growth was restricted to the surface layers (0-4cm), root hair development was considerably less than J2 plants and plants were not as well nodulated.
The majority of plants collected from the J2 site showed symptoms of root disease, in contrast with the relatively healthy J1 plants. It is likely the disease infection was a secondary, physiological response to the more ‘hostile’ soil conditions (for example, acidity and waterlogging) at J2. The lower incidence of infection observed in plants from J1 suggests that the higher pH in the root zone may have improved the ‘health’ of those plants and made them less susceptible to damage and infection. The plants were not screened for specific root diseases, but there are likely to be many different species present such as Pythium, Rhizoctonia, Fusarium and Phytophthora (K Lindbeck pers. comm.).
The Junee paddock is gently undulating and has a history of lucerne pasture, canola and wheat production. A 2013 soil pHCa test result from this paddock of 5.4 for the 0-10cm soil depth failed to detect the variability in soil acidity across the paddock. The blanket lime rate of 1.3t/ha, applied in 2011, but not incorporated (all subsequent crops being direct drilled with a knife point press wheel seeder) was inadequate to ameliorate the severe acidity at 5-15cm at the J2 site.
The effect of acidic layers on root development and nodulation of chickpea
The test strips of prilled lime established by the grower at the Woodstock sites provided an opportunity to determine the effect of soil acidity on chickpea.
The composite 0-10cm soil pH readings from samples at the W1 (prilled lime applied at 290kg/ha) and W2 sites (nil lime) were pHCa of 5.6 and 5.2, respectively (Table 2). Finer sampling of topsoil layers of W2 indicate that at a recommended sowing depth of 5-7cm, seed and rhizobia would have been placed in a moderately acidic layer (pHCa 4.8). Incorporation of the prilled lime at the W1 site created a more suitable soil environment for the rhizobia and young seedling, with a pHCa of 5.45.
Although the nodulation score for the plants growing in the nil lime strips (W2) indicated poor nodulation (a nodulation score of 15.05 compared with 20.7 at the W1 site), the W2 test strips did not show the obvious associated clinical symptoms, N deficiency and restricted root growth observed in affected faba bean crops. It was only when plant roots were inspected and scored for nodulation and root growth and then compared with those from the limed strips (W1) that the impact of the more acidic layers below 5.0cm became clear.
Root growth, root hair development and nodulation were apparently improved by the lime treatment when the sites were first inspected in August. Nodule number and weight was 23% and 36% greater, respectively, on plants from the W1 strips compared with W2 plants. In addition plants from W1 strips showed very little evidence of root disease. In contrast root disease was evident on most plants sampled from W2. The increased susceptibility of chickpea seedlings growing in acidic soils to disease infection was similar to that observed in faba bean at the J2 site.
Table 2. The pHCa measurements of test strips at Woodstock showing the effect of incorporation of 290kg/ha of prilled lime drilled to a depth of 10cm in the seeding rows. Nodulation scores, plant appearance and nodule number and weight reflect the different soil conditions.
| Soil depth (cm) | Woodstock 1 site (+ Lime 2016) | Woodstock 2 site (Nil Lime 2016) | ||
|---|---|---|---|---|
| Soil pHCa | Soil pHCa | |||
| Composite sample | Sub samples | Composite sample | Sub samples | |
| 0-2.5 | 5.60 | 5.93 | 5.21 | 5.87 |
| 2.5-5.0 | 5.81 | 5.36 | ||
| 5.0-7.5 | 5.45 | 4.82 | ||
| 7.5-10.0 | 5.19 | 4.59 | ||
| 10.0-15.0 | 4.89 | 4.72 | 4.80 | 4.67 |
| 15.0-20.0 | 5.05 | 4.91 | ||
| Plant appearance | Plants generally healthy and vigorous, good root development (Aug and Nov sampling). Roots more dense, abundance of root hairs compared with W2 plants. In Nov. roots and nodules concentrated in surface 10-12 cm. Minimal evidence of root disease or discolouration. |
Aug and Nov - shoot growth similar to W1 plants, but root growth relatively poor – shorter, less dense, fewer root hairs. Aug - root disease evident on most plants. Infection not severe enough to cause death of plants, but root pruning and discolouration was evident at Nov sampling. Nov - roots and nodules concentrated in surface 6 cm. |
||
By November 2016 chickpeas from W1 and W2 strips were flowering and had reached a height of about 40cm. Again there were no obvious differences in shoot growth between the test strips, but differences between the W1 and W2 strips were obvious when the roots were inspected.
The depth and density of roots, root hair abundance and nodulation was superior for plants from the W1 strips, obviously benefiting from a pHCa ranging from 5.93 at 0-2.5cm to 5.19 at 7.5-10cm. Roots and nodules extended to a depth of 10-12cm. Conditions at the site were extremely wet from July to October, therefore it is not possible to conclude if root development was restricted to the surface 12cm as a result of intermittent waterlogging or the moderately acid layer at 10-15cm (pHCa of 4.72). It is probable that, even under the more favourable pH conditions at the W1 site, it was the compounding effects of these environmental stresses that adversely affected root development.
In contrast, the roots of the W2 plants were restricted to the surface 6cm; root hair and nodule numbers were considerably less than on W1 plants. The pHCa of the surface 5cm (5.87 at 0-2.5cm and 5.36 at 2.5-5.0cm) appeared to have been satisfactory for development of the surface roots and nodulation, but increased acidity from 5 to 10cm (4.82 at 5.0-7.5cm and 4.59 at 7.5-10cm) restricted root growth and nodule development in these moderately acidic layers. The disease observed on W2 in August was still present in November.
Differences in the appearance and nodulation of chickpea roots suggest a response to the addition of incorporated prilled lime in the seeding row. Yield comparisons for the test strips are not yet available.
Other factors affecting nodulation and early growth of pulses
Management practices that caused severe damage to pulse crops monitored in 2015 and 2016 included:- Crop damage caused by sulfonyl urea (SU) herbicide applied in the previous 12 months. Ineffective incorporation of lime produces an elevated pH in the surface soil layers and delays the breakdown of sulfonyl urea herbicides, for example triasulfuron.
- Addition of zinc to inoculant slurries during the inoculation process. Zinc is toxic to rhizobia and when mixed with the inoculant resulted in extremely poor nodulation. If zinc is to be used on pulses, ensure it is not placed in close proximity to the rhizobia at the time of sowing (application of the zinc in fertiliser mix or separated from seed) or applied to the crop as a foliar application.
Conclusion
Effective nodulation underpins productive and profitable pulse crops. When detailed soil pH data were aligned to root growth and nodulation of pulse crops, it was concluded that the presence of previously undetected, but severely acidic layers was likely to be a major factor responsible for inconsistent ‘performance’ of acid-sensitive pulses on slightly (pHCa >5.0) and moderately acidic soils (pHCa 4.6 to 5.0) of the medium and high rainfall zones.
The impact of acidic layers on root hair development was apparent in all reported crops. As discussed by Drew et al (2012) the main pathway for rhizobial infection of commonly grown temperate legume species is via root hairs. The exception is lupin. At all sites recording poor nodulation (Hb, Ky, J2 and W2), the seed and rhizobia encountered an acidic layer (pHCa 4.4, 4.5, 4.2 and 4.8, respectively) at 5.0- 7.5cm in the profile, which appears to have been sufficient to disrupt the infection process. Optimal nodulation requires pH conditions favourable to both the rhizobia and host plant. These results suggest that although management strategies such as lime pelleting of seed to raise pH in the immediate vicinity of the seed may improve rhizobial activity in the short term, pelleting is unlikely to improve soil pH sufficiently to improve root hair development and significantly improve nodulation.
While most growers are effectively managing disease and weeds in pulse crops and are sowing recommended varieties, our findings highlight the need to review basic agronomic principles and management practices. Management of pulses sown in acidic soils must focus on promoting the nodulation process and minimising or avoiding environmental stresses. The results from this project, reinforced by grower experience, indicate that well nodulated vigorous pulse crops have the ability to withstand multiple stresses, including infection by root diseases and transient waterlogging. The 2015 and 2016 experiences indicate that timely sowing, early in the recommended sowing window, allows plants and nodules to establish before cold temperatures slows growth and rhizobial function. Quoting one of the collaborating growers:
‘Variety is not as important as agronomy… it’s clear from what we are seeing that we need to pay more attention to soils and agronomy’.
Faba bean is proving to be ‘the canary in the coal mine’ for detecting acidic layers. The dramatic clinical symptoms expressed by faba bean plants exposed to acidic layers has helped highlight the extent and severity of pH stratification, even in soils with a long history of lime application. From observations at the Woodstock site, shoot growth of other acid-sensitive species such as chickpea (and most likely lentil, canola and lucerne) does not demonstrate the dramatic response to acidic layers or obvious clinical symptoms exhibited by faba bean. Close inspection of the roots in conjunction with finer soil sampling to check for the presence of acidic subsurface layers is recommended for these crops.
The traditional 0-10cm soil sampling procedure is not detecting pH stratification. Finer sampling at 5cm intervals to a depth of 20cm is needed to verify the presence and depth of acidic layers.
The negative impact on agricultural production of shallow subsurface acidic soil layers is well documented. The presence of these layers effectively places a ceiling on production potential and reduces efficiencies in water and nutrient use. The severity of the acidic layers identified in this study suggests that relatively acid-tolerant species, including barley, canola, lucerne and many wheat varieties are probably suffering a yield penalty at pHCa < 4.7 in the top 15cm.
The intensity of soil pH stratification identified by testing finer layers demonstrates that lime was concentrated in the shallow surface layers (0-5cm) under the no-till systems adopted by the majority of participating growers. This study indicates that current acidic soil management and liming programs are ineffective in neutralising subsurface acidity or counteracting acidification below the shallow surface layers. Lime rates, frequency and method of lime application need to be reviewed in order to improve productivity of acid-sensitive species on acidic soils. A rapid solution to severely or moderately acidic layers at 5-15cm (5-20cm in sandy soils) requires an aggressive approach, including lime incorporation to 10cm with full cultivation. In the longer term appropriate lime rates that maintain pHCa > 5.5 in the entire surface 10cm will facilitate movement of the lime effect into the acidic layers at 10-15cm and avoid further acidification of the 10-20cm layers.
Useful resources
Soil acidity holds back pulse potential - GRDC
Upjohn B, Fenton G, Conyers M (2005) Soil acidity and liming. Agfact AC.19 NSW Department of Primary Industries, Orange.
References
Anon (1991) Field Guide to Nodulation and Nitrogen Fixation Assessment, British Columbia Ministry of Forests.
Anon (2015) Pulse Australia, 2015 Southern Faba & Broad bean – Best Management Practices Training Course Manual.
Conyers, M.K. and Davey, B.G. (1988). Observations on some routine methods for soil pH determination. Soil Science 145, 29-36.
Conyers MK and Scott BJ (1989) The influence of surface incorporated lime on subsurface acidity. Australian Journal of Experimental Agriculture 29, 201-207.
Cregan P and Scott B (1998) Soil Acidification - An Agricultural and Environmental Problem. In ‘Agriculture and the environmental imperative’ . (Eds JE Pratley, A Robertson). pp. 98-128. (CSIRO Publishing: Melbourne)
Drew E, Herridge D, Ballard R, O’Hara G, Deaker R, Denton M, Yates R, Gemell G, Hartley E, Phillips L, Seymour N, Howieson J and Ballard N (2012) Inoculating Legumes: a practical guide. Grains Research and Development Corporation.
Paul KI, Black S and Conyers MK (2003) Development of acidic subsurface layers of soil under various management systems. Advances in Agronomy 78, 187-213.
Richards M, Gaynor L (2016) Southern NSW Pulse Survey 2015-16. NSW Department of Primary Industries, Wagga Wagga
Robertson M, Kirkegaard J, Peake A, Creelman Z, Bell L, Lilley J, Zhang H, Kleven S, Duff C, Lawes R and Riffkin P (2016) Trends in grain production and yield gaps in the high-rainfall zone of southern Australia. Crop& Pasture Science 67, 921-937.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the author would like to thank them for their continued support.
The contribution of the 21 growers participating in this project and their willingness to share their experience is also greatly appreciated.
Contact details
Helen Burns
NSW Department of Primary Industries
Wagga Wagga Agricultural Institute
Pine Gully Road, Wagga Wagga 2650
(02) 6938 1947
helen.burns@dpi.nsw.gov.au
Mark Norton
NSW Department of Primary Industries
Pine Gully Road, Wagga Wagga 2650
(02) 6938 1934
mark.norton@dpi.nsw.gov.au
GRDC Project Code: DAN00191,
Was this page helpful?
YOUR FEEDBACK
