Natural and environmentally friendly way of managing weeds
Author: Yoshiharu Fujii - Professor, Tokyo University of Agriculture and Technology, Japan | Date: 07 May 2014
Natural and environmentally friendly way of managing weeds without herbicides
Yoshiharu FUJII, Professor, Tokyo University of Agriculture and Technology, Japan
Key Messages
To study allelopathy, we have developed specific bioassays, namely, “Plant Box Method, “Sandwich Method” and procedure to isolate allelochemicals. Using these methods, we have evaluated about 4,000 plants from Japan, Asia and Pacific countries.
A new concept called “Phytolark”, an ideal ground cover plant with allelopathic activity provides natural and environmentally friendly way of managing weeds without herbicides.
We isolated innovative bioactive natural chemicals from allelochemicals and used allelopathic ground cover plants to control weeds in agriculture and natural systems. For example, we isolated L-DOPA from velvet bean (Mucuna pruriens), cyanamide from hairy vetch (Vicia villosa), rutin from buckwheat (Fagopyrum esculentum), tryptophan from Centipede grass (Eremochloa ophiuroides), salicylic acid from dwarf mondo grass (Ophiopogon japonicus), militarine from hyacinth orchid (Bletilla striata), lycorine from red spider lily (Lycoris radiate),; and cis-cinnamic acid from Thunberg spiraea (Spiraea thunbergii).
We have been using allelopathy to managing weeds in an environmentally friendly way without synthetic herbicides.
Aims
Allelopathy is a phenomenon whereby a plant influences their neighbouring plants, insects, microorganism, and animals by natural chemicals called allelochemicals.
We have developed specific bioassays for allelopathy, namely, “Plant Box Method”; “Sandwich Method”; “Dish-pack Method”; and “Rhizosphere soil Method”. Using these methods, we have evaluated about 4,000 plants from Japan, Asia and Pacific countries.
Our main aims are 1) to isolate innovative bioactive natural chemicals from allelochemicals, and 2) to use allelopathic ground cover plants in agriculture and environment. In this regard, I would like to propose a new concept called “Phytolark”, an ideal ground cover plant with allelopathic activity that could also be a natural resource for food, medicinal use, or landscape (i.e. with beautiful flowers, leaves or fragrant perfume). Phytolark provides natural and environmentally friendly way of managing weeds without herbicides.
Method
Allelopathy is a complex concept and studying allelopathy is not always easy for in-depth understanding and isolation of the natural chemicals causing the allelopathy. We developed various methods to effectively study allelopathy in various plant parts of the potential allelopathy plant species and isolation of the allelochemicals. The methods are 1) Assessment of allelopathic activity by the plant-box method, 2) Assessment of allelopathic activity by the sandwich method, and 3) Procedures for isolation of allelochemicals and evaluation of weed suppression.
Results
Assessment of allelopathic activity by the plant-box method
Among the 70 leguminous species tested, velvet bean (Mucuna pruriens) showed the strongest inhibitory activity. We isolated L-3,4-dihydroxypenylalanine (L-DOPA) as a major allelochemical from velvet bean. Among its cultivars, ‘hassjo’, semi-dwarf type from Japan was the strongest. Vicia species such as Vicia faba1, a winter legume crop cultivated for food and cover crop in Japan and the USA and Vicia villosa1, known as hairy vetch or woolly pod vetch, also used as a cover crop in the USA was found allelopathic. We isolated cyanamide as a new allelochemical from Vicia species. Other promising legumes are Medicago1 spp, Leucaena leucosephala (Lead tree)1, Canavalia ensiformis (Jackbean)1, Melilotus spp (Melilots)1, Puerarila lobata, and Vigna1 spp.
For the allelopathic activity of Gramineae species, this family showed lesser inhibitory effects than the leguminous species. Avena spp like Oat (Avena sativa) known for its allelopathic activity since ancient times showed the strongest inhibition with wild oats, A. sterillis, A. murphy, and A. barbata showing the highest activity. Other Gramineae species that seem promising are Setaria italica, Panicum miliaceum, Anthoxanthum odoratum, Triticum spp, Sorghum spp, and Hordeum spp. Most of these species have been reported as allelopathic plants.
Among the Compositae species, Artemisia absinthium1 and A. princeps, both medicinal herbs, were found remarkably allelopathic, but lesser compared to Leguminous and Gramineous family. Lettuce (Lactuca sativa), the acceptor plant used in this bioassay, belongs to the same family as A. absinthium. This could be the likely reason of its reduced sensitivity. Additionally, the Compositae family, contain hydrophobic chemicals and these compounds could not migrate under the plant-box condition where the medium is water based.
Sulphur compound in the Cruciferae family, Brassica1 family, is allelopathic. Some of these compounds such as isothiocyanates are well known to inhibit the growth of soil-born fungi and bacteria. These compounds are also inhibitory to plants. Using this family as a cover crop, soil-born diseases can be reduced in sustainable agriculture. Broccoli and cauliflower are the strongest in this group. How to use these vegetables for weed suppression, poses a challenge.
The results from the Labiatae family, well known for the production of essential oils, showed that some plants known as herbs, such as lavender1 (Lavandula angustifolia), oregano (Origanum vulgare), clary (Salvia sclarea) (Mintweed family)1, and savory (Satureja hortensis) are inhibitory.
The Amaranthaceae1 family is well known for its plant smothering effect in the fields of onion, carrot and itself, but showed only slight inhibition in our test. It is therefore not clear if its weed suppression capability is due to allelopathy through root exudates or not. Further testing using acceptor plants other than lettuce perhaps could elucidate its contribution to allelopathy if there is any. Some Amaranthus spp are promising ground cover crops that suppress weeds in tropical countries.
In the Cucurbitaceae family, there are several important vegetables but only Lagenaria siceraria var. hispida and Cucurbita maxima were slightly allelopathic. These plants with large leaf systems usually inhibit the growth of weeds once established since they cover the surface of the land completely. Combination of competition for light and allelochemical factors including volatile compounds may be responsible for the complete suppression of weeds.
In Liliaceae, Lycoris radiata showed allelopathic activity. This plant was introduced into Japan more than 1500 years ago from China and distributed by farmers into their footpaths of rice paddy fields and graveyards. Consequently the Japanese people call this plant ‘flowers of the dead’. Rats and moles shy away from this plant because it produces toxic alkaloids in its tubers. These chemicals may play an important role as allelochemicals for defense.
Most of the Caryophyllaceae family showed no allelopathic activity. As for other families, velvet leaf (Abutilon theophrasti)1, already reported very allelopathic, likewise showed strong allelopathic inhibition in our test, but the active chemicals remain unknown. Ruta graveolens (Rue) and Symphytum spp. (Comfrey) were also found allelopathic and so were the ground cover plant, Portulaca oleracea1, or common purslane, and some members of this group producing beautiful flowers.
Based on this method, plants such as Avena, Mucuna, Abtilon, Erigeron, Brassica and Vicia that have been reported allelopathic have likewise shown strong inhibitory activity by the plant-box method.
Assessment of allelopathic activity by the sandwich method
These methods evaluate the allelopathy through fallen leaves from trees, crops and weeds. Leaf litter leachates play an important role in natural conditions, and in practical farming systems such as mulching by leaf litter. The principle of our developed method is to elute the chemical compounds from leaves placed between the two layers of agar, and referred to as the “sandwich method”.
“Sandwich method” evaluated allelopathy of leaf materials of 2,000 plant species in the world. For the evaluation of allelopathic activity, we introduced the concept of “standard deviation value”. Among the species tested, lemongrass1 (Cymbopogon citratus) and derris (Derris scandens) showed the strongest inhibitory activity and caused 100% growth inhibition in the radicle and hypocotyl in lettuce seedlings. Betel pepper (Piper betle), tamarind1 (Tamarindus indica), and gliricidia1 (Gliricidia sepium) also showed strong inhibitory activity. All these plants are known to have specific natural chemicals and used as herb, medicine, or special use for insect control.
Laboratory and greenhouse experiments were conducted to evaluate the allelopathic potential of dwarf lily-turf (Ophiopogon japonicus) on lettuce, alfalfa, timothy and rape plant growth. Dry leaf debris, aqueous extract of fresh leaves and O. japonicus grown soil were investigated. Emergence, dry weight and root and shoot length of all bioassay species were inhibited in concentration dependent fashion when grown in soil incorporated with oven-dried leaves of O. japonicus. However, the degree of inhibition varied among the test plant species. The aqueous leaf extract was highly phytotoxic and it significantly reduced germination, seedling growth, and fresh weight of all test species. The active chemical in O. japonicus was isolated as beta-sitosterol, p-hydroxy benzoic acid, and salicylic acid (m-hydroxy benzoic acid). Among these compounds, salicylic acid was most active and contained about 0.03%. It was concluded that this compound is responsible for the allelopathy.
Isolation of allelochemicals and evaluation of weed suppression
As for allelochemicals from buckwheat (Fagopyrum esculentum) and tartary buckwheat (Fagopyrum tataricum), bioactivity guided purification and identification by Mass and NMR spectroscopy have yielded various allelochemicals that has catechol structure. Most important allelochemicals in buckwheat was rutin. Field trials carried out at experimental fields showed strong weed inhibition by buckwheat. Plots where buckwheat was grown mixed with weeds, produced more than 75 % reduced weed biomass as compared to buckwheat free plots. These findings suggest that buckwheat can be used as cover crop as the allelochemicals released from the living or decaying plant material can reduce the growth of various co-occurring weeds effectively.
We isolated L-DOPA from velvet bean (Mucuna pruriens), cyanamide from hairy vetch (Vicia villosa), rutin from buckwheat (Fagopyrum esculentum), tryptophan from Centipede grass (Eremochloa ophiuroides), salicylic acid from dwarf mondo grass (Ophiopogon japonicus), militarine from hyacinth orchid (Bletilla striata), lycorine from red spider lily (Lycoris radiate), and cis-cinnamic acid from Thunberg spiraea (Spiraea thunbergii). The chemical structure of some allelochemical molecules are given in Figure 1.
The compounds isolated as allelochemicals with potent activity are important precursor for new bioactive chemicals as herbicide or organic farming. Velvet bean, hairy vetch or sunflower is useful also as ground cover crops. It is of much value to recommend these allelopathic crops in the sustainable agriculture for the safe weed and pest control in the world.
Conclusion
Allelopathy is a phenomenon whereby a plant influences their neighbouring plants, insects, microorganism, and animals by natural chemicals called allelochemicals. Many allelopathic plant species exist in each agricultural region. These allelopathic plants should be characterised to understand how to minimise their harmful effects on crop plants and maximise their effects in controlling weeds to reduce pressure on herbicides. Natural chemicals from plant species are of great use in organic farming and also in situation where herbicide resistance is the worst.
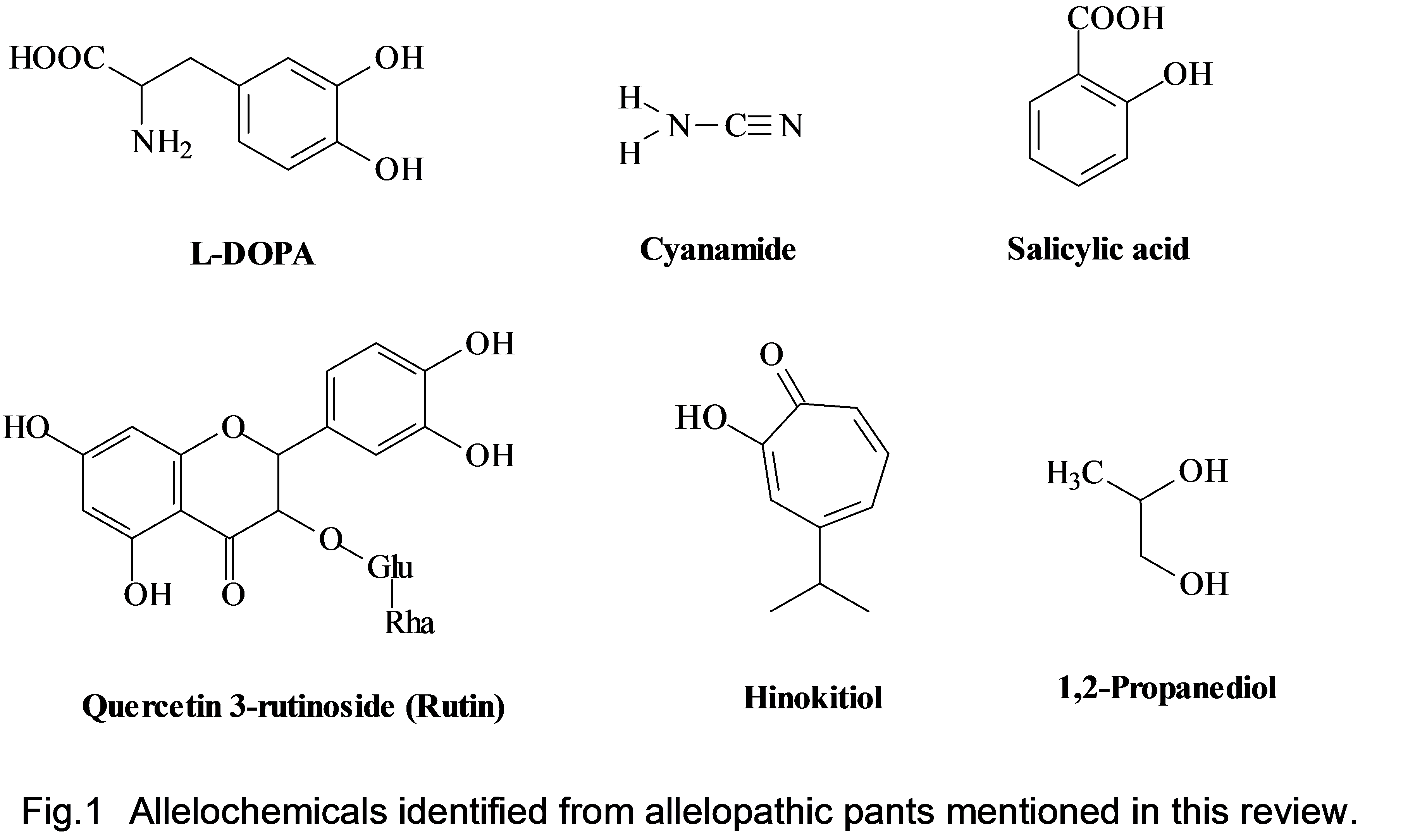
Key words
Allelopathy, methods to study allelopathy, allelopathic plant species, allelochemicals, natural weed control, ground cover plant.
Acknowledgments
I gratefully acknowledge Department of Agriculture and Food Western Australia for sponsoring my travel to Perth as a Visiting Specialist and providing the opportunities to exchange knowledge and ideas with the scientists and agronomists here. I do also acknowledge GIWA for cooperation.
GRDC Project No.: Not applicable
Paper reviewed by: Dr Abul Hashem
Was this page helpful?
YOUR FEEDBACK
