Background
Nitrogen is the key major nutrient influencing crop production in Australian agricultural systems, and maintaining a close balance between inputs and outputs as well as better synchronization between N supply and plant demand is the role of soil management, fertiliser, crop residues and crops.
Fertiliser N use in Australia increased at an annual rate of approximately 14 per cent compared to that in 1992 which is not only considered economically unsustainable but also environmentally undesirable. Most of the N-cycling processes such as N fixation, mineralization and gaseous loss are biologically mediated. A diverse group of microbial communities are involved in the release of nitrate N from SOM and they are present in all agricultural soils. There are direct links between processes involved in soil organic N cycling that also play a role in the transformation of fertiliser nitrogen (Figure 1). Therefore, management strategies that manipulate microbial communities controlling organic N cycling would help optimise both organic and fertiliser N use efficiency.
Soil type, crop rotation and management practices associated with tillage, stubble retention and fertiliser application can influence the diversity of microbial populations and along with the environment they affect biological processes involved in nitrogen fixation, mineralization, and availability and losses. All of these processes and the associated microorganisms can be manipulated to optimise N-use efficiency both by improving the supply of N from organic N and decreasing the losses via denitrification and leaching.
Nitrogen mineralized from soil organic matter and crop residues contributes to a large part of crop N requirements in the rainfed cropping regions across southern Australia. For example, in the year of application, fertiliser N contributes approximately 20-40 per cent of the total N supply of wheat. Soil N supply comes from soil organic matter and recent crop residues and the rate of supply is influenced by the soil biological capacity and modulated by management and environmental factors.
The nitrogen mineralization potential of the top 10 cm soils generally ranges from 10-35kg N/ha/season in sandy, 25-70 kg N/ha/year in clay and loam soils, and 30-100kg N/ha/year in red brown earth soils.
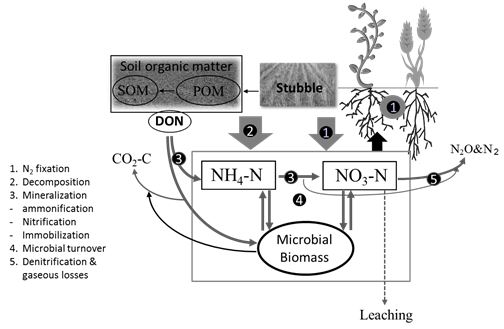
Figure 1: Biological processes involved in nitrogen cycling that influence plant available nitrogen levels in soil. SOM – soil organic matter, DON – dissolved organic nitrogen, POM – particulate organic matter.
The magnitude of soil biological processes and their impact within the farming system varies seasonally due to the variation in the time of their occurrence relative to the crop growth and demand (Gupta et al. 2011). The effect of soil organisms involved in N mineralisation can be seen in both the off-season (fallow) and in-crop season (Figure 2). Nitrogen mineralised during the off-season may accumulate and/or be lost through leaching, denitrification or weed uptake, whereas the N mineralised during the growing season in the rhizosphere may be utilised immediately by the crop. In a farming system, factors influencing nutrient mineralisation–immobilisation processes need to be understood in order to synchronise nutrient availability to plant needs and also to reduce nutrient losses. Additionally, critical periods of biological activity must be taken into consideration to optimise management strategies that help synchronise N supply to availability to crops.
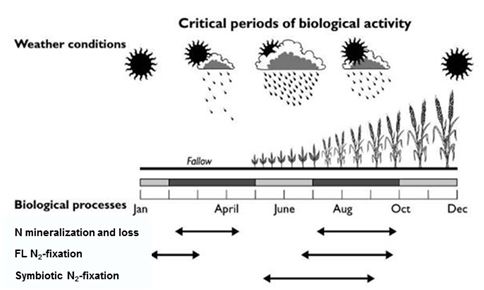
Figure 2: A conceptual diagram showing functionally important periods for different N-cycling biological processes and their impact within the farming systems in Australian winter-cropping growing regions.
Decomposition and N mineralization
In low fertility Australian agricultural soils, crop residues are one of the major sources of carbon (C) for soil biota and retention of stubble after harvest contributes to the conservation of nutrients taken up by the plant within the cropping system. A large portion of N used by crops is mineralized from previous crop and pasture residues through the activity of soil microorganisms (microbial biomass, MB). Decomposition of crop residues is mainly a biological process involving diverse groups of microbial communities and facilitated by the activity of soil fauna. Land use changes from mixed farms where crop rotation with legume pastures was common to continuous cereal cropping generally resulted in a decline crop residue based N mineralization (Angus et al. 2006). The decline occurred mainly through altered crop residue quality, e.g. wider C:N ratio (100:1) cereal residues replacing N-rich legume residues (15 to 25:1). It is considered that crop residues with a C:N ratio >22:1 generally result in immobilisation (tie-up) of mineral N in microbial biomass. The rate and timing of availability of nutrients from stubble to the following crops is determined by the rate of decomposition and immobilisation (tie-up) by soil microorganisms (N in microbial biomass; MB-N). The amount of N in microbial biomass varies with soil type, crop rotation, tillage and other management practices that can influence microbial populations (Table 1). In southern Australian cropping regions, the effect of loss of nutrients from stubble removal may be greater than the temporary tie-up of the nutrients during decomposition when retained. However, the scale of these effects vary depending upon stubble load, time and type of burning and tillage.
Table 1: Amount of nitrogen in microbial biomass and the soil N supply potential as influenced by soil type.
| Location |
Soil type |
Microbial biomass
(kg N/ha) |
N supply potential
(kg N/ha)5 |
|---|
| Waikerie/Karoonda,SA |
Sand and sandy loam |
25-45 |
10-35 |
| Streaky Bay, SA |
Calcarosol |
30-60 |
15-50 |
| Kerrabee, NSW |
Loam |
60-75 |
35-50 |
| Temora, NSW |
Red earth |
75-105 |
50-100 |
| Rutherglen, Vic |
Red brown earth |
50-100 |
30-100 |
| Leeton/Warialda, NSW |
Clay |
50-110 |
25-75 |
$ N supply potential is calculated from N in MB plus N mineralization measured in a lab-incubation assay.
Stubble retention can provide benefits through changes in soil's physical, chemical and biological properties. However, the selection of stubble management strategy would have a substantial impact on the potential benefits to be gained from the activity of soil biota in their role in carbon turnover, nutrient mineralisation, and subsequent availability of nutrients to crops. For example, tillage practices accelerate the decomposition and microbial turnover resulting in quick accumulation of mineral N, especially in soils with lower microbial biomass levels. In addition, research from Victoria and South Australia (SA) has shown that tillage can disrupt the linkages between the activity of microbes processing organic N and those related to fertiliser and mineral N transformations influencing the rate of release and accumulation of mineral N in soil (Phillips et al. 2015; Gupta et al. 2011). This means strategic tillage practices could be developed to manipulate N release and losses (especially from legume residues), for example to synchronise the release of N to plant demand and avoid losses through leaching and denitrification.
Nitrogen released during decomposition and soil organic matter turnover is rapidly assimilated by MB which is subsequently released through microbial turnover and microbe-fauna interactions. Results from field experiments in SA indicated that in the sandy soils in the Mallee with lower levels of MB, there can be substantial movement (leaching) of mineral N (25-50kg N/ha; P<0.05) down the soil profile following summer rainfall (Figure 3). Retention of stubble which generally increases the amount of MB can therefore arrest the leaching of mineral N to lower depths.
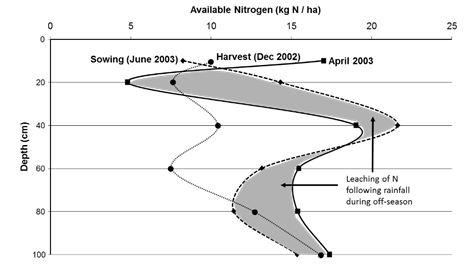
Figure 3: Leaching of mineral N in the soil profile following summer rainfall in a continuous cropping treatment at Waikerie, SA (soil type – Mallee sand).
Research at Karoonda in SA, on a dune-swale landscape, has shown that plant type (e.g. wheat, cereal rye, canola or pasture) can cause large changes in the functional diversity of microorganisms, i.e. microbial communities involved in various biological functions including N cycling processes. Thus, in a crop rotation, such changes coupled with differences in the quantity and quality of organic residues (tops and roots) can significantly modify the N mineralization-immobilization processes and availability of N (McBeath et al. 2014). The magnitudes of these effects vary with soil type and region which needs to be considered when designing fertiliser N management strategy in a cropping sequence.
Nitrogen fixation - free-living N fixation
Biological nitrogen fixation, by symbiotic and free-living (FL) bacteria can provide economic and environmental sustainability to N management in Australian agriculture. Free living N fixation refers to N fixation by bacteria growing independently in soil or in close association with plant roots where symbiotic N fixation occurs through legume-rhizobia interaction in nodules. Research has shown that communities of free-living and endophytic N fixing bacteria have been found in association with cereal crops, grasses (including summer active perennial pastures) and non-leguminous plants. With the increased adoption of intensive cropping and area under consecutive cereal crops (>50%), FL-N fixation has the potential to make a major contribution to N requirements in cereal crops.
Additionally, current day conservation farming systems support a habitat that promotes activity of FL-N fixing (nifH gene harbouring) bacterial communities both during off-season and in crop, i.e. increased microsites with C availability, wide C/N ratio etc. Improvements in FL-N fixing capacity in soils can provide multiple benefits through reduced requirement for N inputs, disease suppression, C sequestration, etc.
Estimates of FL-N fixation, measured using a laboratory based incubation (15N isotope) assay, ranged from <0.15 to 2.3kg N fixed/ha/day under optimal soil moisture and temperature conditions. FL-N fixation ranged from 0.2 to 1.5kg N/ha/day in sand and sandy loam soils in low to medium rainfall regions of southern and Western Australia compared to 0.5 to 2kg/ha/day in the clay and loam soils in high rainfall regions. The number of optimal days per season does vary in different agricultural regions. The amount of N fixed varied with soil type and influenced by the time of sampling (in crop versus non-crop/fallow period), crop type and mineral nitrogen levels. The amount of FL-N fixed during summer significantly increases (>50%, P<0.05) in the presence of summer-active grasses such as Rhodes grass and Panicum species, compared to winter-cereal crop only systems (Figure 4; Gupta et al. 2014).
The abundance of FL-N fixing bacteria, percentage clay content (soil type), soil moisture content and carbon availability are some of the major factors influencing FL-N fixation in cropping soils. Therefore, removal of stubble (one of the major sources of available C) either by burning or grazing would have negative impact on the amount of N fixed by FL-N2 fixing bacteria. Research in the southern Australian agricultural region has shown that FL-N2 fixation was higher immediately after harvest and decreased as summer progresses (Figure 4). Thus, careful consideration should be given to how stubble is managed in order to maximize FL-N fixation in cropping soils. Free-living N fixation is generally higher soon after rainfall when the water content is adequate to provide the required low-oxygen conditions (to protect O2-sensitive N fixing enzymes) and carry the carbon to where these bacteria are located. Higher levels of mineral N in the surface soil (0-10cm) could have a negative effect on the amount of fixation by free-living bacteria, but this varies with soil type so needs region-specific solutions.
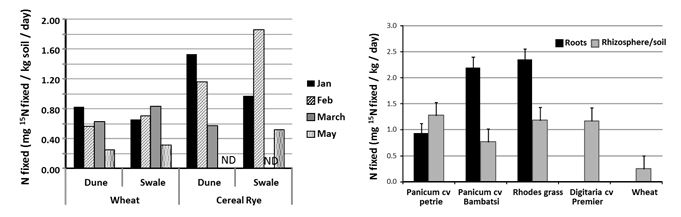
Figure 4: Amount of FL-N fixation in soils collected from field experiment at Karoonda in SA during summer of 2011/12 (left) and with summer-active perennial grasses (right).
Soil type and stubble retention have a large influence on the abundance of nifH-gene harbouring bacteria, for example abundance increased with clay content (P<0.01) and stubble retention (P<0.05). Populations of FL-N fixing bacteria are generally higher in the rhizosphere soil (soil closely surrounding roots) are generally higher than those found in the bulk soil.
Genetic profiling of N2-fixing bacteria (nifH gene sequencing analysis) in cereal crop field soils (from Qld, NSW, SA and WA) indicated the presence of a diverse group of free-living community (112 genera) in different agricultural regions indicating differences based on soil type and environment. Crop and variety types can influence the abundances of various groups thereby affecting the amount of FL-N fixation. Further research could suggest specific management strategies and identify crop varieties that help promote FL-N fixation by specific communities of N2-fixing bacteria in different soils and regions.
Denitrification and gaseous N losses
The composition and abundance of soil bacteria involved in gaseous N losses (e.g. denitrification and nitrification) varies with soil type, and the denitrification losses are highest where soil nitrate N levels are high and when sufficient biologically available C is present along with low oxygen (O2) concentrations e.g. water logging. In the southern Australian cropping regions, N losses are sporadic in time and space and vary widely in different agricultural systems (Grace et al. 2015). In cropping soils, the primary consideration for reducing gaseous N losses is by matching the supply of mineral N to crop demand and management practices that promote tie-up of N in microbial biomass (immobilization) generally reduce N losses both through denitrification and leaching.
Nitrification of N fertilisers
The conversion of ammonia and urea N found in commonly used N fertilisers into nitrate N is a biological process mediated by specific group of microorganisms, e.g. nitrifiers which are mostly abundant in the surface soils. The abundance and the type of nitrifiers present varies with soil type and depth and their activity can be influenced by management practices. Research has shown that banding fertilisers can influence the activity of these microbes and the accumulation of nitrate N (Angus et al. 2014). Thus, fertiliser N use efficiency could be manipulated by targeting fertiliser placement or the use of nitrification inhibitors. Immobilization of fertiliser N in MB, becoming unavailable to plants, is generally short-term and has been found to be available to crops later in the crop season or to the following crop provided it is not leached or lost through gaseous losses.
Conclusion
- Nitrogen mineralized from SOM and crop residues makes a large contribution to crop N uptake (>50%)
- A diverse group of microbial communities are involved in the release of nitrate N from SOM and they are present in all agricultural soils
- Management strategies (such as stubble retention, tillage, fertiliser application and green manuring) and crop and variety selection can help manipulate microbial communities involved in N mineralization from organic matter and crop residues and also influence fertiliser N use efficiency
- Free-living N fixation can make agronomically important contribution to the available N pool in stubble retained cereal based systems and perennial grass systems.
Useful resources
Angus, J.F. et al. (2006) N mineralization in relation to previous crops and pastures. Soil Research. 44: 355-365.
Angus, J.F. et al. (2014) Effects of banded ammonia and urea fertilizer on soil properties and the growth and yield of wheat. Crop and Pasture. 65: 337-352.
Gupta, V.V.S.R. et al. (2011) Principles and management of soil biological factors for sustainable rainfed farming systems. In: Rainfed farming systems’ by P. Tow, et al., pp. 149-184, Springer Sci. & Business Media.
Gupta et al. (2014) Nitrogen cycling in summer active perennial grass systems in South Australia: Non-Symbiotic N fixation. Crop & Pasture Science 65: 1044-1056.
McBeath, T.M., Gupta, V.V.S.R., et al. (2015) Break Crop Effects on Wheat Production across Soils and Seasons in a Semi-Arid Environment. Crop and Pasture Science. 66: 576-579.
Phillips, L.A. et al. (2015) Organic nitrogen cycling microbial communities are abundant in dry Australian agricultural soil. Soil Biology Biochemistry. 86: 201-211.
Acknowledgement
Funding for this work was provided through the GRDC Projects MSF0003, CSP00186 and CSP00138 and their support gratefully acknowledged.
Contact details
Vadakattu Gupta
CSIRO Agriculture, Waite campus
08 8303 8579




