The mobility of P in alkaline clays of the Northern Grains Region
Author: Karl Andersson, Matt Tighe, Chris Guppy, Paul Milham and Tim McLaren, UNE | Date: 01 Mar 2016
Take home message
- The reserve-P (the P extractable by BSES in excess of Colwell-P) in alkaline soils is made up of mineral and sorbed forms.
- The concentration of reserve-P extracted with an anion exchange membrane (AEM) to mimic root uptake increased sharply at pH 6. This pH is achievable by natural processes of acidification in the soil close to active roots (the rhizosphere). The dissolution of CaP by acidification may be at least as important to P nutrition as slow dissolution.
- The release of reserve-P, through acidification and the extraction of both P and Ca in the laboratory, may explain field observations of rhizosphere acidification and the depletion of reserve-P by crops in neutral-alkaline agricultural soils.
- The ongoing availability of CaP in soils is likely influenced by the form of reserve-P, the capacity of plants to acidify the rhizosphere, and the pH buffer capacity of the soil.
- The improved understanding of the release of reserve-P can help improve agronomic options e.g. species selection and fertiliser rates.
Introduction
The alkaline Vertosols of the northern grains region contain a pool of phosphorus (P) that is rapidly available to plants, and a diminishing reserve-P fraction that has been considered to slowly replenish the available pool. The process of replenishment is not well understood, and this may lead to inefficient use of fertiliser P. Bell et al. (2014) have provided guidelines for Colwell-P and BSES-P to indicate likely responses to the application of P fertiliser at depth. The reserve-P is acid-soluble, and may be native soil P or fertiliser reaction products. The difficulty in estimating the supply of reserve-P to labile-P is illustrated by the weaker correlation between BSES-P and Colwell-P in soils with substantial reserve-P compared to those with low reserve-P (Moody et al., 2013). McLaren et al. (2014) proposed that reserve-P can contribute to the slow replenishment of the soil solution above that of Colwell-P when the Ca:P in the BSES extract is less than 57:1 (on a molar basis). Crop response both with and without P fertilisation in Vertosols (e.g. Dalal, 1997; Pundarikakshudu, 1989; Solis and Torrent, 1989; Wang et al., 2007 and other soils (Hinsinger and Gilkes, 1995; Johnston and Poulton, 1992; Ziadi et al., 2001) have shown that plants access acid-soluble P not measured by the bicarbonate method. It is possible that modification of soil in the rhizosphere, particularly acidification, enables plants to directly access the acid-soluble P fraction.
Methods
We analysed grey or black vertosols for common properties, including pH, EC, Colwell-P, BSES-P, P buffer index (PBI), oxalate extractable Fe, Al and P, exchangeable cations, pH buffer capacity, and carbon content (both inorganic and organic). We mostly focussed on soils with moderate to high concentrations of reserve-P (> 300 mg kg–1). The soils had low PBI, were non-sodic and non-saline, and contained 0 to 0.3% carbonate.
We mimicked rhizosphere processes to study the release P from these soils. The technique used repeated extraction of P from soil suspensions while manipulating the pH. We measured the concentration of P that accumulated in solution after 18 h, P that was removed with AEM, or P that was removed with AEM while Ca was also removed with both AEM and cation exchange membranes (ACEM). These steps were repeated 15 to 20 times, while we manipulated the pH of the suspensions so it gradually acidified to ~pH 4, was held the initial pH, or was gradually acidified then held at pH 6.5 or pH 5.5. Finally, we analysed the forms of P in the untreated and extracted soil with bulk P K-edge X-ray absorption near edge spectroscopy (XANES). The XANES measurements were made at SLRI in Thailand.
Results and discussion
Incremental acidification
The results presented are for three soils: soils 1 and 2 contain moderate concentrations of reserve-P (~300 mg kg–1), and soil 3 contains high reserve-P soil (6600 mg kg–1). Following the early removal of labile P sources, P recovery remained low until soil pH passed key thresholds (Figure 1). In contrast, previous investigations involving repeated extractions, but without manipulating pH, have found diminishing concentrations of P with increasing extraction time. These thresholds varied little between soils, though soil pH buffer capacity affected the amount of acid required to approach the thresholds. With the solution P concentration kept below 1 µM using an AEM, the threshold was pH 6.0–6.3 (AEM-P), i.e. within the range of potential rhizosphere pH. When released P was instead allowed to accumulate in solution (DI-P), a similar release of P occurred, but at pH 5.3–5.5. The concentrations extracted decreased as reserve-P was depleted (soils 1 and 2), or continued to increase where reserve-P remained in the soil (soil 3). Soils 1 and 2 had similar concentrations of reserve-P, but soil 1 contained a larger proportion in a more readily solubilised form. This indicates that the particular form of reserve-P influences its solubility and availability.
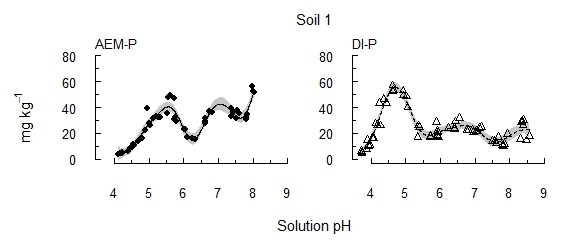
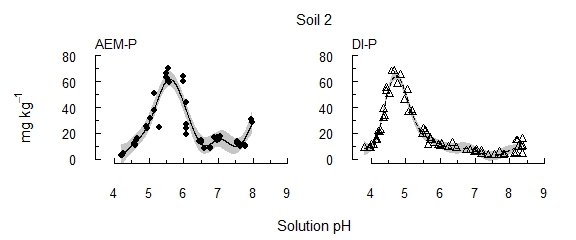
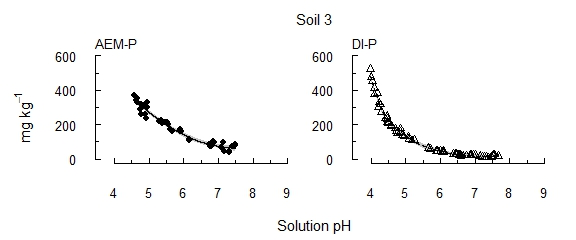
Figure 1. The concentration of P solubilised from each of three alkaline vertosols as pH was incrementally acidified.
Static pH
When the pH was either maintained at the initial level (pHi), or lowered to and held at pH 6.5 or 5.5, more P was released at each lower pH value (Figure 2). As the target pH was established, the concentrations of P extracted with AEM were similar to the concentrations removed at the equivalent pH during the incremental acidification. Pulses of P were released by acidification to pH 6.5 and then to pH 5.5, suggesting multiples pools of different solubility. Importantly, differences in the solubility of reserve-P were again evident between the soils. The co-removal of P and Ca from solution with ACEM, released approximately twice as much P from each soil in the initial two to three extractions compared to the AEM alone (Figure 2). While reserve-P remained in the soils, greater concentrations of P were extracted with the ACEM as the pH 6.5 and pH 5.5 targets were established than with the AEM.
The equivalent concentration of Colwell-P was depleted early from each soil (Figure 3). Some reserve-P was extracted at pHi, supporting the concept of slow replenishment of Colwell-P by reserve-P. However, the higher concentrations of P released by acidification of the soils after Colwell-P was removed show the importance of pH to the release of reserve-P. Since plant roots take up both P and Ca, and can acidify the rhizosphere, the results suggest that plants may directly access the acid-soluble P fraction.
Analysis by XANES identified that reserve-P includes CaP mineral phases and sorbed P phases, and that their contribution to the P extracted varied with pH. The mineral phases identified were apatite in each soil, along with the more soluble brushite in two moderate-P soils and octacalcium phosphate in the high-P soil. Acidification to pH 6.5, with AEM in the moderate P soils and ACEM in the high P soil, depleted the more soluble CaP minerals. Further acidification to pH 5.5 also depleted apatite. Smectite-sorbed P, identified in each soil, was depleted with P extraction at each pH level. While only low concentrations of P continued to be extracted at pH 5.5, other sorbed pools were identified which comprise part of the total soil P in excess of the reserve-P. The remaining pools are likely to be less available to plants, and indicate that the BSES-P extraction is a useful indicator of extractable P.
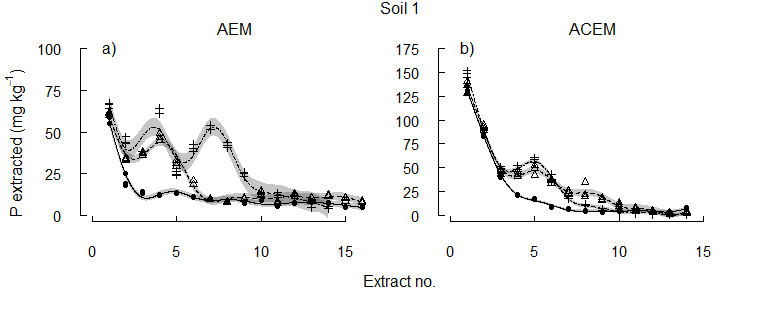
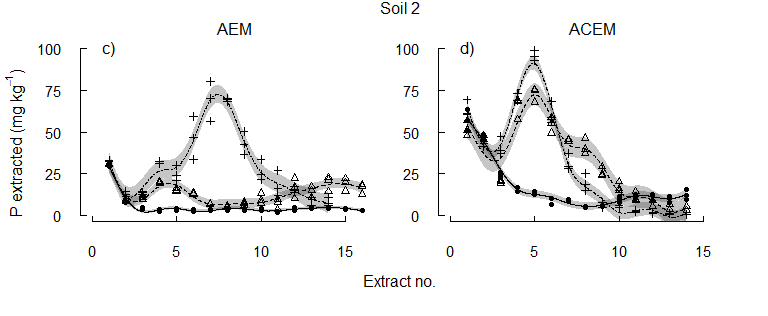

Figure 2. The concentration of P solubilised from each of three alkaline vertosols as pH was maintained at the initial soil pH, pH 6.5, or pH 5.5.
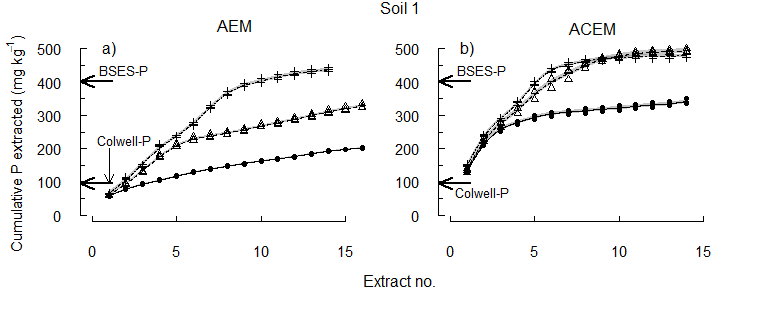
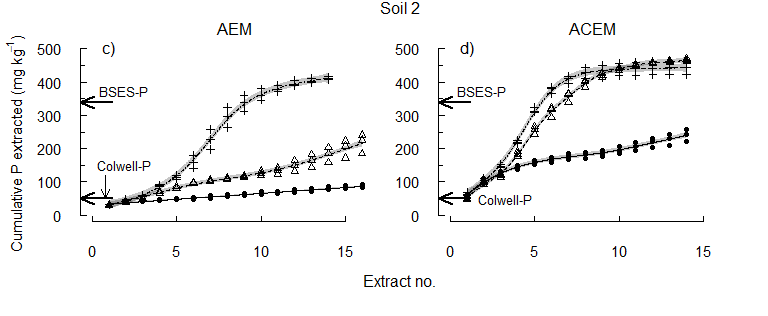
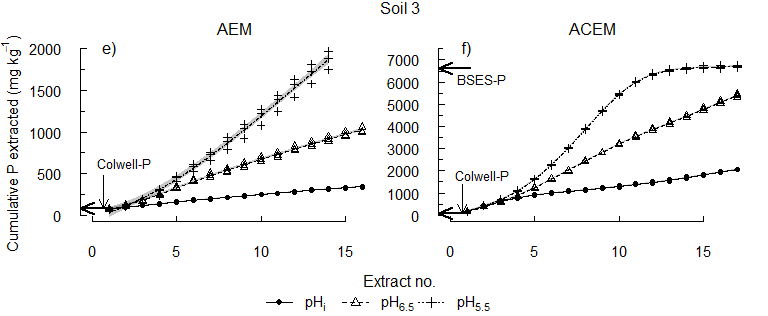
Figure 3. The cumulative concentration of P extracted at pHi, pH 6.5 or pH 5.5.
Conclusion
The findings suggest that the depletion of acid-soluble P previously observed in agricultural systems on Vertosols may be driven, at least in part, by modification of the soil conditions. Even though rhizosphere acidification is energy expensive for plants, acidification may nonetheless be the process behind the depletion of acid soluble reserve-P in the Vertosols of the northern grains region. Broader understanding of the pH buffering properties of the soils, and the nature of P reserves, may allow better selection of varieties and management of P fertiliser.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through the support of the GRDC, the authors would like to thank them for their continued support. We also acknowledge the support of the Australian Postgraduate Award, The Australian Synchrotron, and the Thai Synchrotron (SLRI). Results from this research have been published in Geoderma. Figures 1 is derived from Andersson et al. (2015), and Figures 2 and 3 are from Andersson et al. (2016).
References
Andersson, K.O., Tighe, M.K., Guppy, C.N., Milham, P.J., McLaren, T.I., 2015. Incremental acidification reveals phosphorus release dynamics in alkaline vertic soils. Geoderma 259-260, 35-44. DOI: http://dx.doi.org/10.1016/j.geoderma.2015.05.001
Andersson, K.O., Tighe, M.K., Guppy, C.N., Milham, P.J., McLaren, T.I., 2016. The release of phosphorus in alkaline vertic soils as influenced by pH and by anion and cation sinks. Geoderma 264, 17-27. DOI: http://dx.doi.org/10.1016/j.geoderma.2015.10.001
Bell, M., Lester, D., Power, B., Zull, A., Cox, H., McMullen, G., Laycock, J., 2014. Changing nutrient management strategies in response to declining background fertility: The economics of deep Phosphorus use, GRDC Grains Research Updates. Grains Research Development Corporation, Coonabarabran, pp. 30-38.
Dalal, R.C., 1997. Long-term phosphorus trends in Vertisols under continuous cereal cropping. Soil Res. 35(2), 327-340. DOI: http://dx.doi.org/10.1071/S96052
Hinsinger, P., Gilkes, R.J., 1995. Root-induced dissolution of phosphate rock in the rhizosphere of lupins grown in alkaline soil. Soil Res. 33(3), 477-489. DOI: http://dx.doi.org/10.1071/SR9950477
Johnston, A.E., Poulton, P.R., 1992. The role of phosphorus in crop production and soil fertility: 150 years of field experiments at Rothamsted, United Kingdom. In: J.J. Schultz (Ed.), Phosphate fertilizers and the environment. International Fertilizer Development Center, Muscle Shoals, Alabama, USA, pp. 45–64
McLaren, T.I., Guppy, C.N., Tighe, M.K., Moody, P., Bell, M., 2014. Dilute acid extraction is a useful indicator of the supply of slowly available phosphorus in Vertisols. Soil Sci. Soc. Am. J. 78(1), 139-146. DOI: http://dx.doi.org/10.2136/sssaj2013.05.0188
Moody, P.W., Speirs, S.D., Scott, B.J., Mason, S.D., 2013. Soil phosphorus tests I: What soil phosphorus pools and processes do they measure? Crop Pasture Sci. 64(5), 461-468. DOI: http://dx.doi.org/10.1071/CP13112
Pundarikakshudu, R., 1989. Studies of the phosphate dynamics in a Vertisol in relation to the yield and nutrient uptake of rainfed cotton. Exp. Agric. 25(01), 39-45. DOI: http://dx.doi.org/10.1017/S0014479700016422
Solis, P., Torrent, J., 1989. Phosphate fractions in calcareous vertisols and inceptisols of Spain. Soil Sci. Soc. Am. J. 53(2), 462-466. DOI: http://dx.doi.org/10.2136/sssaj1989.03615995005300020026x
Wang, X., Lester, D.W., Guppy, C.N., Lockwood, P.V., Tang, C., 2007. Changes in phosphorus fractions at various soil depths following long-term P fertiliser application on a Black Vertosol from south-eastern Queensland. Soil Res. 45(7), 524-532. DOI: http://dx.doi.org/10.1071/SR07069
Ziadi, N., Simard, R.R., Tran, T.S., Allard, G., 2001. Soil-available phosphorus as evaluated by desorption techniques and chemical extractions. Can. J. Soil Sci. 81(2), 167-174. DOI: http://dx.doi.org/10.4141/s00-040
Contact details
Karl AnderssonSchool of Environment and Rural Science
University of New England, Armidale
Ph: 6773 3497
Email: kanderss@myune.edu.au
GRDC Project Code: GRS10029,
Was this page helpful?
YOUR FEEDBACK
