The invisible threat: Canola yield losses caused by root lesion nematodes in WA
Author: Sarah Collins, Carla Wilkinson, Sean Kelly, Helen Hunter, Lucy DeBrincat, Karyn Reeves and Kefei Chen, DAFWA South Perth | Date: 28 Feb 2017
Key messages
- Canola yields were significantly reduced (average 16% yield loss) by medium to high root lesion nematode (P. neglectus or P. quasitereoides) populations at sowing.
- Variety selection reduces potential yield impacts caused by root lesion nematodes, but where starting levels are medium to high, substantial yield loss may occur for all varieties of TT canola tested.
- Lupins are resistant (reduce nematode densities) and tolerant (reduced yield loss in the presence of RLN) to medium to high populations of P. neglectus and P. quasitereoides and can be used as a break crop to reduce nematode populations in a poor yielding paddock.
Background and aims
Root lesion nematodes (RLN; Pratylenchus species) are microscopic pests that feed on the roots of crop plants; subsequent damage to root systems can cause significant yield loss. In the Western region, RLN are widespread across the grain belt (Figure 1) and losses are potentially large. In 2016, 63% of NVT paddocks sampled in WA were infested with RLN; 83% of infested paddocks contained P. neglectus and 23% P. quasitereoides. Potential yield losses in cereals can be up to 50% if beginning of season levels are >15 RLN/g soil.
RLN are managed through the cultivation of resistant crops (which reduce nematode densities) and/or tolerant crops (which have reduced yield loss in the presence of RLN). Increased understanding of the effects of rotational crops on RLN densities improves our ability to determine their potential effects on subsequent cereal crops. Likewise an understanding of the RLN tolerance of current wheat, barley, pulse and oilseed varieties helps estimate the potential economic effects of RLNs in the Western region and aids in appropriate choices for infested paddocks.
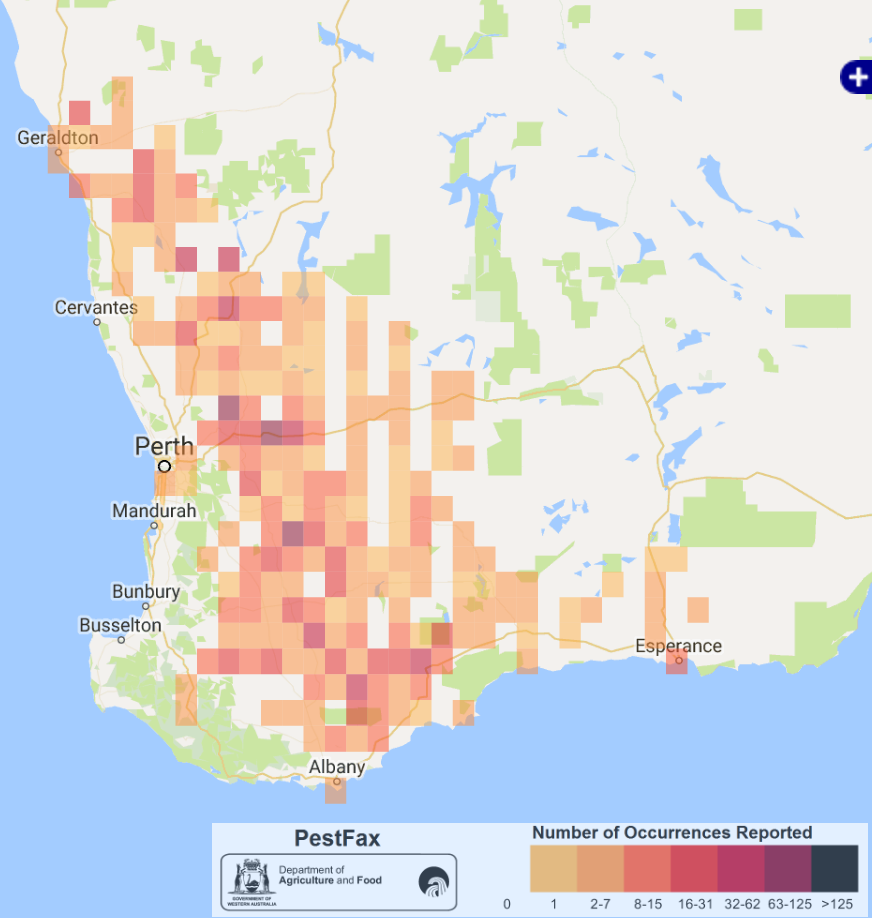
Figure 1a
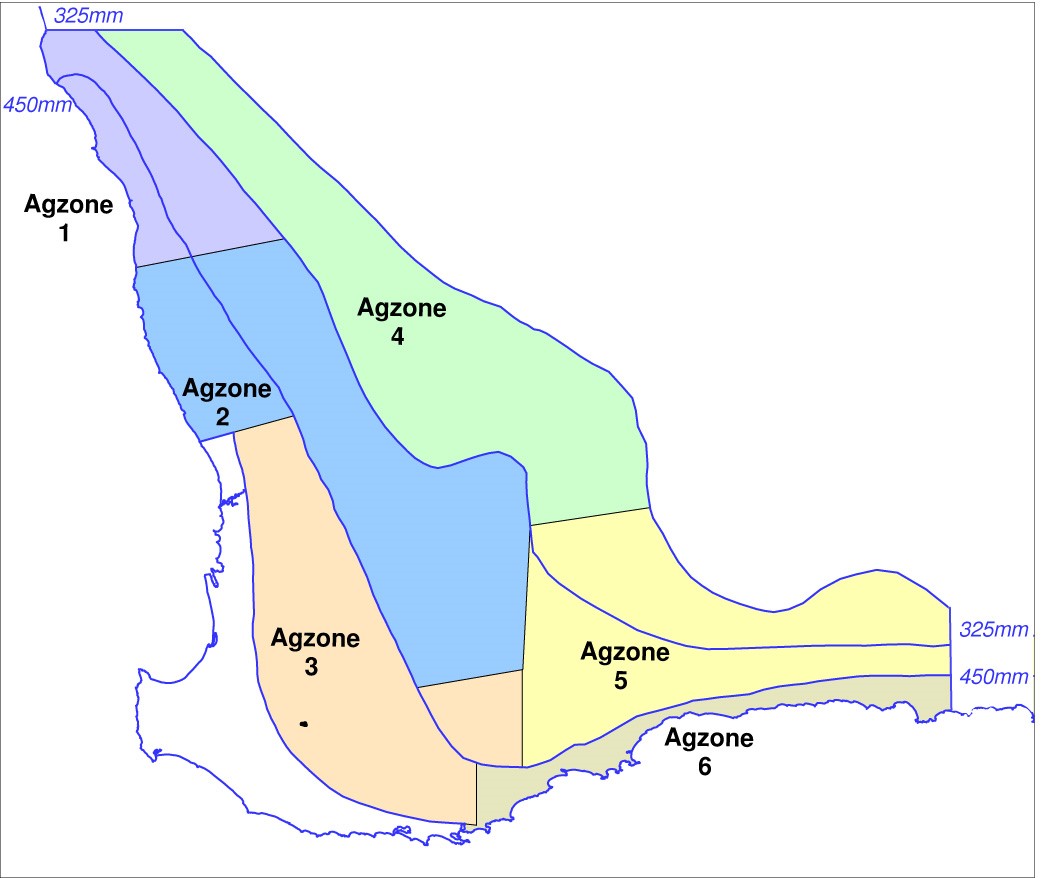
Figure 1b
Figure 1. Broadacre cropping area for the Western Region of Australia showing a) recorded distribution of root lesion nematode (Pratylenchus) (DAFWA Pestfax Maps) and b) zoning based on rainfall and regional location (DAFWA Geographic Information Services Maps).
In cereal crops paddock symptoms include uneven or wavy growth, less tillering, yellowing of the lower leaves and early wilting. Examination of infested cereal roots will show brown lesions as well as a reduction in the number of lateral roots. Visible RLN symptoms have not been observed in canola plants hence it has been assumed that canola yields, although moderately susceptible, were not impacted by RLN infestation. However, with an increasing utilisation of canola in western region cropping rotations and increasing RLN levels in the Western region (Collins et al. 2015) research was undertaken to determine the field resistance and tolerance of canola.
A series of trials are underway across the grain-belt investigating the impacts of the three RLN species known to cause major yield losses in the Western region; P. neglectus, P. quasitereoides, and P. penetrans. A range of WA specific crops, varieties and agro-ecological zones (Figure 1) are targeted so that an array of WA growing conditions are represented in RLN research.
This paper describes the resistance (impact of crop on nematode population) and tolerance (impact of nematode on crop yield) of triazine tolerant (TT) canola varieties to root lesion nematodes Pratylenchus neglectus and P. quasitereoides in different Western Region Agzones. These are the first yield loss trials of this kind conducted in WA. While numerous wheat, barley and lupin varieties were also tested in these trials, results are only shown at a crop level as the aim of this paper is to show the relative field resistance of canola to other commonly grown crops and the resistance and tolerance of different canola varieties.
Method
The Western region’s most commonly grown grain crops; wheat, barley, canola and lupins are investigated in this field research series which commenced in 2015 and ends in 2017. To develop information applicable across the region, the trial series is also conducted across a range of Agzones (Figure 1). The trials under discussion also exemplify rainfall areas where the differing RLN species are commonly known to cause yield loss; Medium rainfall zone Wongan Hills (Agzone 2; P. neglectus) and High rainfall zone Gibson (Agzone 6; P. quasitereoides).
Suitable variety entries were determined from canola National Variety Trials information (Bucat et al. 2016). Triazine tolerant (TT) canola varieties were utilised because they constitute the majority of the area sown to canola annually in the Western region (Bucat et al. 2016). Each trial was sown in naturally infested paddocks using a split plot design oversowing blocks where RLN populations had been manipulated into ‘high’ (medium to high) and ‘low’ (low to medium) densities in the previous season (using provisional yield loss risk categories as a guide (Table 1)). Other confounding soilborne pathogens were negligible as determined by the SARDI qPCR at commencement of the trial. RLN densities were measured in each plot at the beginning (Pi) and end (Pf) of season to determine field multiplication (variety resistance information). Sampling was conducted by collecting 400 g soil samples on row from each plot using a CSBP soil corer (20 cores/plot). Soil samples were tested by SARDI qPCR analysis for RLN numbers/g of soil (Hollaway et al. 2003). Plots were harvested and yield was compared between ‘low’ and ‘medium’ nematode plots using analysis of variance ANOVA procedure to determine if RLN had impacted yields (tolerance information).
Table 1. Western region provisional yield loss risk categories (McKay et al. 2016).
|
Pratylenchus level |
Pratylenchus/g soil |
% yield loss |
|---|---|---|
|
Below Detection Level |
<0.1 |
<2 |
|
Low |
0.1–<2.0 |
0–15 |
|
Medium |
2.0–<15 |
0–30 |
|
High |
≥15 |
0–50 |
Results
Crop resistance to RLN (nematode impact)
Canola was susceptible to both species of RLN tested (multiplication > 1); the P. neglectus population increased five-fold (Wongan Hills) to an average of 45 RLN/g soil while P. quasitereoides increased three-fold (Gibson) to 12 RLN/g soil during the growing season (Table 2). When compared to the other crops tested, the degree of RLN multiplication in canola varied between RLN species. For P. neglectus there were significant differences in multiplication between crops with wheat most susceptible, then barley, followed by canola, and lupin proving to be resistant to RLN multiplication (Figure 2a). For P. quasitereoides, lupins were resistant (multiplication <1), but there was no statistical difference in the resistance of wheat, barley and canola (Figure 2b).
Table 2. Average Pratylenchus neglectus and P. quasitereoides multiplication and final RLN (/g soil) levels in TT canola varieties assessed in Wongan Hills and Gibson, respectively. All plots combined for each variety.
|
Variety |
P. neglectus (Wongan Hills) |
P. quasitereoides (Gibson) |
||||||
|---|---|---|---|---|---|---|---|---|
|
Pi* |
Pf⌂ |
Multiplication |
Pi* |
Pf⌂ |
Multiplication |
|||
|
ATR Cobbler |
11 |
45 |
3.7 |
a◊ |
6 |
10 |
3.2 |
a◊ |
|
ATR Snapper |
10 |
40 |
4.2 |
ab |
8 |
14 |
2.7 |
a |
|
Telfer TT |
8 |
41 |
4.4 |
ab |
5 |
12 |
3.6 |
a |
|
Sturt (TT) |
10 |
49 |
4.9 |
ab |
5 |
12 |
3.2 |
a |
|
ATR Stingray |
11 |
53 |
5.6 |
b |
5 |
13 |
2.9 |
a |
|
Av. |
10 |
45 |
4.6 |
6 |
12 |
3.1 |
||
Note. *Pi = average RLN level at the beginning of season. ⌂Pf = average RLN level at the end of season. ◊ = Different letters denote significant difference in multiplication between varieties from multiple comparisons (at the significance level of 0.05) using Fisher’s Protected LSD.
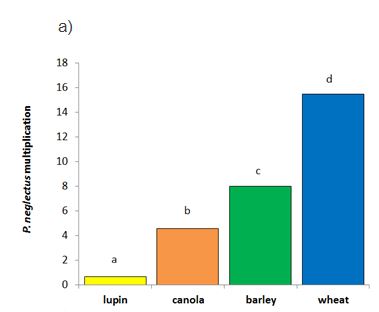
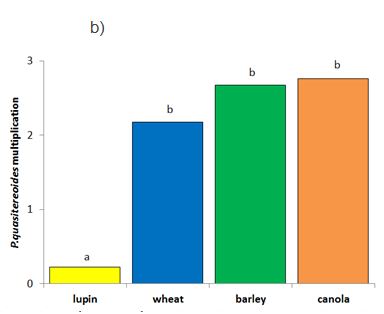
Figure 2. Multiplication of P. neglectus (Wongan Hills, 2015) and P. quasitereoides (Gibson, 2015) in four crop types. Different letters denote significant difference in multiplication between crops within a RLN species from multiple comparisons (at the significance level of 0.05) using Fisher’s Protected LSD.
Canola variety resistance to RLN (nematode impact)
All canola varieties increased the RLN levels over the season but the degree of RLN multiplication was affected by the variety grown. For P neglectus, ATR Stingray was the most susceptible variety and increased the RLN population significantly (p<0.05) more during the season than ATR Cobbler (Table 2) with multiplication rates of 3.7 (Pf = 45 RLN/g soil) compared to 5.6 (Pf = 53 RLN/g soil) respectively. P. quasitereoides multiplication was not significantly different between canola varieties indicating that there was no difference in resistance between the varieties tested.
Canola tolerance to RLN (yield impact)
When RLN levels were low in plots at the time of sowing, average canola yields were 1.72 t/ha and 2.53 t/ha at Gibson and Wongan Hills, respectively. ATR Stingray and ATR Snapper were the highest yielding varieties at both sites (Table 3) when RLN pressure was low. Sturt (TT) average yield was substantially lower than these varieties at Wongan Hills and was also lower than all other TT canola varieties tested in the Gibson trial (Table 3).
Table 3. Canola yield at low RLN levels at Gibson (1 RLN/g soil) and Wongan Hills (3 RLN/g soil) trial sites, 2015.
|
Variety |
Gibson (t/ha) |
Wongan Hills (t/ha) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
ATR Stingray |
1.98 |
a* |
2.77 |
a |
||||||
|
ATR Snapper |
1.83 |
ab |
2.70 |
a |
||||||
|
ATR Cobbler |
1.72 |
b |
2.44 |
b |
||||||
|
Telfer TT |
1.66 |
b |
2.40 |
b |
||||||
|
Sturt (TT) |
1.39 |
c |
2.35 |
b |
||||||
|
Av. |
1.72 (Agzone 6; 1.52) |
2.53 (Agzone 2; 1.45) |
||||||||
* Different letters denote significant (p<0.05) difference in yield at low RLN levels between varieties from multiple comparisons (at the significance level of 0.05) using Fisher’s Unprotected LSD.
Yield impacts caused by RLN infestation were large with an average loss of 16% at both Wongan Hills (398 kg/ha) and Gibson (275 kg/ha) due to P. neglectus and P. quasitereoides, respectively. Yields of all varieties were reduced when RLN was medium to high at the time of sowing; average of 17 P. neglectus/g soil at Wongan Hills and 11 P. quasitereoides/g soil at Gibson. All yield impacts were significant (p <0.05), with the exception of Telfer TT at the Gibson trial. Variety tolerance to RLN was not consistent between RLN species, but at both sites, ATR Stingray was the best performing variety where RLN levels were low at the time of sowing, yielding 2.77 and 1.98 t/ha at Wongan Hills and Gibson, respectively (Figure 3). In contrast, Sturt (TT) had the lowest yields in both trials, irrespective of the level of RLN present (Figure 3). Further results are split according to RLN species.
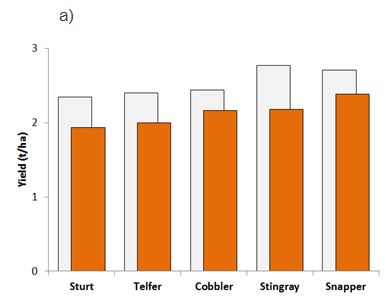
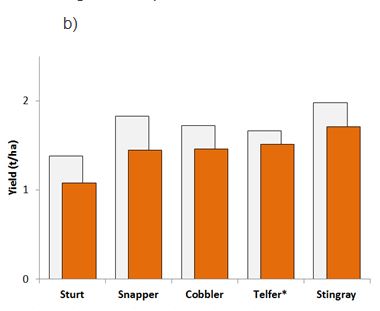
Figure 3. Grain yields for five canola varieties commonly grown in the Western Region with either ‘low’ (grey bars) or ‘medium’ (orange bars) beginning of season RLN levels in corresponding plots at trials conducted for a) P. neglectus (av. Pi 3 vs 17 RLN/g soil); or b) P. quasitereoides (av. Pi 5 vs 11 RLN/g soil). * denotes cultivar impacts that were not significantly different (p>0.05).
Variety tolerance to P. neglectus
All canola varieties sustained yield loss (p<0.05) due to high P. neglectus populations at sowing, (Figure 3a) with 11% to 21% yield reductions. ATR Stingray was the highest yielding variety when RLN was low but was the most intolerant variety with a loss of 21% (590 kg/ha) when P. neglectus populations were high (Figure 3a). ATR Snapper was the highest yielding variety in the presence of high nematodes at 2.38t/ha while losing 12% yield (320 kg/ha) compared to low RLN plots. ATR Cobbler was the most tolerant variety tested, with 11% yield penalty (270 kg/ha) (Figure 3a).
Variety tolerance to P. quasitereoides
Variety yields were 9 to 22% lower in plots that had medium P. quasitereoides levels at the beginning of the season. Sturt (TT) and ATR Snapper were least tolerant to RLN infestation, suffering the greatest yield impacts of 308 kg/ha (22%) and 385 kg/ha (21%), respectively. ATR Stingray had the highest yield, even after 14% (271 kg/ha) loss in the presence of medium P. quasitereoides at the time of sowing (Figure 3b).
Conclusion
Canola experienced substantial yield losses due to root lesion nematodes P. neglectus and P. quasitereoides in these trials. Unfortunately, this indicates that these RLN species can cause yield loss in all of the broadacre crops most commonly grown in the western region; wheat, barley and canola. In the concurrent trials conducted at the Wongan Hills and Gibson sites, yield loss in canola was highest and consistent with an average of 16% yield loss caused by both RLN species tested. Losses in cereal cultivars were more variable, ranging from no yield loss to 15% yield loss in wheat and no yield loss to 7% in barley. In our trial series lupins have been consistently resistant to both P. neglectus and P. quasitereoides and its yield was not affected confirming that lupin is a reliable break crop for these RLN species.
Crop resistance varied between the RLN species tested but followed the same general trends as the yield loss potentials. Lupin varieties reduced the RLN populations over the season and are therefore a good choice to manage RLN when it is at levels that could cause significant yield loss in other crops. For paddocks infested with P. quasitereoides, barley, canola and wheat are equally susceptible and will most likely increase nematode levels. In paddocks infested with P. neglectus, canola is less susceptible than barley, which is in turn less susceptible than wheat. For both RLN species there are differences in susceptibility between cultivars which can be utilised by growers to limit RLN build up to prevent potential yield loss in grain crops. For wheat and barley, resistance and susceptibility information can be found in local variety guides and can be used to help growers make rotational choices in infested paddocks.
All five canola varieties tested were impacted by P. neglectus and P. quasitereoides, with yield losses of 9% to over 20% in the most intolerant varieties. The field trials were conducted using TT varieties which accounted for 72% of the area sown to canola in the 2015 season. The trials were also carried out in different Agzones (2 and 6), with markedly different climatic characteristics and soil types. Visible symptoms due to infestation from P. neglectus or P. quasitereoides have not been observed in canola plants, so yield loss has remained un-recognised until now. These factors combined suggest that yield loss in canola may be more widespread across the Western region.
Variety selection reduces potential yield impacts caused by RLN, but where starting levels are medium to high, substantial yield loss can be expected for all varieties of TT canola tested. This is the case where either P. neglectus or P. quasitereoides are present in the soil. ATR Stingray for example, was the best performing variety in both trials when RLN levels were low at time of sowing but lost 590 kg/ha (21%) from P. neglectus impacts and 271 kg/ha (14%) from P. quasitereoides where starting numbers were ‘medium’. Further testing is necessary to confirm variety tolerance, particularly for other canola varieties because RLN multiplication and yield loss will vary according to seasonal and environmental conditions, and potential interactions from breeding characteristics developed in different herbicide tolerance systems. All variety yield averages in ‘low’ plots were within the range expected for that Agzone. This suggests that the 2015 season was conducive for canola growth at both sites, but RLN impacts may differ in seasons that are unfavourable for canola crops.
Canola is commonly regarded as a good break crop to reduce soil borne diseases in cereal rotations, particularly where rhizoctonia is an issue (Huberli et al. 2013), but canola is likely to cause an increase in RLN levels. In these concurrent trials, canola increased both P. quasitereoides and P. neglectus levels by an average of 3 to 5 times, reflecting results from previous DAFWA research (Collins et al. 2015). RLN multiplication in canola increased potential for cereal yield loss in the next season to ‘high’ (0 - 50% yield loss) at the Wongan Hills site, according to current cereal yield loss categories for PreDicta B in the western region (McKay et al. 2016). P. quasitereoides at Gibson, also increased, moving from the lower to upper limits of the PreDictaB ‘medium’ (0 – 30% yield loss) rating for potential negative yield impacts in cereals (McKay et al. 2016). Rhizoctonia and RLN symptoms are similar, and the two are often found together in the Western region. This means that growers can inadvertently increase RLN to potentially damaging levels if caution is not taken to identify the cause of problem areas in crops, particularly in cereals where symptoms are visible. These results exemplify the reason that caution should be taken in self-diagnosis of ‘patchiness’ and unthrifty areas in crops so that effective management can be employed in the next season. Testing and monitoring through DAFWA’s Diagnostic Laboratory Services (DDLS)-Plant Pathology (formerly AGWEST Plant Laboratories) or the South Australian Research and Development Institute’s (SARDI) PreDicta B® service, can help to correctly diagnose, monitor and manage nematodes and root and hypocotyl diseases in a range of crops.
References
Bucat J, Seymour M, French B, Malik R, Harries M, and Sprigg S (2016) Canola variety guide for Western Australia 2017. Department of Agriculture and Food Western Australia Bulletin 4877. ISSN No. 183 7366.
Collins SJ, Wilkinson CJ, Kelly SJ, DeBrincat L, and Hunter HL (2015) Plant parasitic nematodes causing strife in Western Australian broadacre crops. In Proceedings of the Australasian Plant Pathology Society 2015 Conference (September, 2015). P. 95.
DAFWA Geographic Information Services Maps (2015) Crop variety testing (CVT) zones of Western Australia. Paper 1. Research Library.
Hollaway GJ, Ophel-Keller KM, Taylor SP, Burns RA, and McKay AC (2003) Effect of soil water content, sampling method and sample storage on the quantification of root lesion nematodes (Pratylenchus spp.) by different methods. Australasian Plant Pathology 32, 73-79.
Huberli D, Connor M, Miyan S, MacLeod W, Desbiolles J, Bogacki P, and McKay A (2013) Integrated disease management options to control rhizoctonia bare-patch in cereals. 2013 Agribusiness Crop Updates, 25 - 26 February, Perth, Western Australia. Research Repository website.
McKay A, Mayfield A, and Rowe S (2016) “Broadacre Soilborne Disease Manual Version 9.0”. The South Australian Research and Development Institute (SARDI), Australia.
National variety trials website (2017).
Acknowledgments
DAFWA Wongan Hills and Esperance RSUs, particularly Shari Dougall and Chis Matthews, provide quality trial management. Jeremy Wish provided expertise for Agricultural Production Systems sIMulator (APSIM) use and interpretation. Jackie Bucat and Daniel Huberli provided valuable review of this paper. The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
Was this page helpful?
YOUR FEEDBACK
