Yellow spot of wheat epidemiology studies
Author: Jean Galloway (1), Ciara Beard (1), Pip Payne (1), Geoff Thomas (1) and Moin Salam (2) | Date: 07 Mar 2017
1 Department of Agriculture and Food Western Australia
2 Freelance Consultant, Bangladesh
Take home message
The fungus that causes yellow spot survives from one season to the next on infected wheat stubble. In the Western Australian (WA) Grainbelt yellow spot fruiting bodies mature and ascospores are released from the previous season’s stubble earlier in the southern coastal cropping areas (Albany and Esperance) compared with central (Northam) and northern (Eradu) cropping areas. The timing of fruiting body maturation is influenced by environmental factors, predominantly rainfall and temperature.
The early maturation of the yellow spot fungus in the southern coastal cropping area, usually before crops have emerged, results in limited primary infection opportunities. This, combined with cooler temperatures, can limit yellow spot impact in most years in this region. The later maturation of the yellow spot fungus in the central and northern cropping areas is often after crop emergence and can result in multiple primary infection opportunities. These multiple primary infection opportunities, coupled with warmer winter conditions which favour secondary spread, result in continuous wheat crops in the northern and central areas having a high risk of developing yellow spot.
The timing of the onset of yellow spot ascospore release from stubble does vary from year to year. In years when the stubble has been exposed to several summer rainfall events the fruiting bodies mature earlier in the autumn compared with years that have had limited rainfall events between harvest and the autumn break. Progress has been made towards developing and validating a decision support tool (DST) that predicts the timing of yellow spot ascospore release. This is being developed and tested with data from WA, SA and Victoria. This DST will assist wheat growers, advisors and researchers make informed decisions about yellow spot management.
Background
The fungus that causes yellow spot (Pyrenophora tritici-repentis) survives from one season to the next on wheat stubble. Sexual spores, called ascospores, develop in fruiting bodies on this stubble and when released infect the following season’s wheat crop; this is the primary infection process. Once the wheat crop is infected, yellow spot lesions on the leaves produce abundant asexual spores, called conidia, causing further infection within the crop canopy; this is the secondary infection process. Both the primary and secondary infection processes are facilitated by rainfall, with disease being more problematic in years with regular growing season rainfall.
In WA we noticed three things about yellow spot. Firstly, we observed that in the southern coastal cropping areas around Albany and Esperance yellow spot was generally not a yield limiting disease. We attributed this observation to it being ‘too cold’ for disease development in this area. Secondly, we knew that yellow spot was more severe in the central and northern cropping areas. Thirdly, we found that in time of spraying trials that the time of disease onset was not consistent and we got variable results from tillering or stem extension sprays.
To gain a better understanding of yellow spot we began detailed epidemiology work to determine how and when spores were released from infected stubble and how this might influence time of disease onset in wheat crops grown over infected stubble.
What we did
We began quantifying the effect of environment on the development of yellow spot fruiting bodies on wheat stubble at Northam in WA in 2008. From 2011-2016 we used a range of locations in the southern coastal cropping area (Albany and Esperance), southern inland cropping area (Katanning), central cropping area (Northam) and northern cropping area (Eradu) of WA. Recently (2014-2016) our locations have been extended to include stubble weathered in SA (Waite and Hart) and Victoria (Horsham).
Stubble infected with yellow spot during the growing season was collected immediately after harvest each year from a single location and in December sub-samples of this stubble was placed on the soil surface at the various locations described above. The stubble was allowed to weather under natural environmental conditions at these locations over the following summer, autumn and winter each year.
Stubble was inspected at fortnightly intervals from the beginning of autumn for fruiting bodies. The maturation stage of the sexual spores (ascospores) within these fruiting bodies was rated using a microscope. From this data the time of onset of maturation and the incidence of fruiting bodies that contained mature spores with the potential to cause primary yellow spot infection was determined.
What we found
Ascospores produced on the previous season’s wheat stubble are the source of primary inoculum that initiates yellow spot outbreaks in WA.
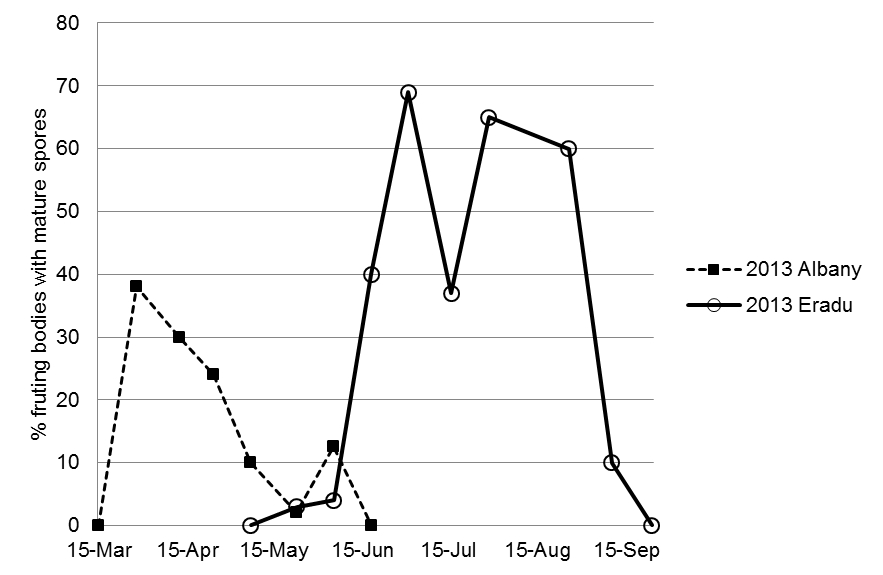
Figure 1. Maturity progress of yellow spot fruiting bodies on wheat stubble at two locations in the WA Grainbelt, Albany (south) and Eradu (north). In the southern coastal cropping area of Albany maturation occurred earlier than in the northern cropping area at Eradu.
In all years we observed that in the cooler, wetter autumn of southern coastal cropping areas yellow spot ascospores mature earlier on the previous season’s stubble compared with the drier, warmer autumn of central and northern cropping areas of the WA Grainbelt (Figure 1).
The ascospore maturity information as shown in Figure 1 above has given us knowledge of when the primary infection windows are occurring. The relationship between the timing of the primary infection window and growth stage of the wheat crop helps explain the timing of observed onset and subsequent severity of yellow spot in various seasons and locations. We can use this to help interpret the results of yellow spot fungicide management trials that were conducted in similar locations. As an example, we can use the 2013 ascospore maturation data from Eradu in the northern cropping area (Figure 1) to interpret the results of a replicated fungicide timing trial conducted in the same cropping region at Eneabba (125 km away) in the same year. This comparison shows that in 2013 the primary infection window for yellow spot at Eradu began in mid-June and covered most of the growing season (Figure 1) and would have allowed for multiple infection opportunities, starting from soon after the crop had emerged. Disease severity data from Eneabba (Table 1) showed that by July 2013 yellow spot was already apparent and that by September high levels of disease had developed in the unsprayed crop.
Table 1. Yellow spot disease progress (% leaf area affected) in the northern cropping area of WA (Eneabba) in 2013.
|
Fungicide Treatment |
Yellow spot disease severity (leaf area diseased) |
||
|
Z31 (17 July) |
Z59 (7 August) |
Z71 (3 Sept) |
|
|
Nil fungicide |
2.2 a |
12.7 a |
58 a |
|
In-furrow + Z59 spray |
0.8 b |
9.2 b |
41 b |
|
Z31 spray + Z59 spray |
2.2 a |
8.3 b |
39 b |
|
Z59 spray |
2.2 a |
12.7 a |
43 b |
|
LSD (5%) |
0.9 |
3.3 |
13.2 |
Values followed by the same letter are not significantly different
Where an in-furrow treatment was applied at sowing on 23 May, this significantly reduced the level of yellow spot compared with the nil fungicide treatment (Table 1). By comparing the disease progress data with the maturation progress (Figure 1) we can see that the in-furrow treatment was present at a perfect time in 2013, the fungicide was active as ascospores were being released from the seedling stage onwards and was able to significantly reduce the number of successful primary infection events. A similar effect can be seen with the tillering (Z31) fungicide spray which significantly reduced the level of yellow spot that developed in the crop compared with the nil fungicide treatment (Table 1). In years where ascospore release is prior to crop emergence or is delayed significantly until later growth stages then early fungicide treatments are less likely to be effective.
The timing of fruiting body maturity and the onset of yellow spot ascospore release does vary significantly from year to year within a location (Table 2). In years when the stubble has been exposed to several summer rainfall events the fruiting bodies mature earlier in the autumn compared with years that have had limited rainfall events between harvest and the autumn break. There is a complex relationship between moisture and temperature associated with fruiting body maturation. Summer rainfall effectively ‘primes’ fruiting bodies but until temperatures reach suitable levels ascospore development within the fruiting bodies is delayed.
Table 2. Timing of onset of yellow spot fruiting body maturity at 8 locations in Australia assessed as number of years in which maturity onset was recorded in each time period. Maturity onset is the first occurrence of one or more fruiting bodies containing mature ascospores capable of initiating primary yellow spot infection.
|
Site |
Time Period |
|||||||||||
|
Late Mar |
Early Apr |
Mid Apr |
Late Apr |
Early May |
Mid May |
Late May |
Early Jun |
Mid Jun |
Late Jun |
Early Jul |
Mid Jul |
|
|
Albany |
1 |
1 |
1 |
0 |
0 |
1 |
0 |
1 |
||||
|
Esperance |
2 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
|||
|
Katanning |
1 |
0 |
2 |
0 |
0 |
1 |
||||||
|
Northam |
1 |
0 |
0 |
1 |
1 |
1 |
3 |
0 |
1 |
|||
|
Eradu |
1 |
0 |
1 |
2 |
1 |
|||||||
|
Waite |
1 |
0 |
0 |
0 |
1 |
|||||||
|
Hart |
1 |
0 |
0 |
0 |
1 |
|||||||
|
Horsham |
1 |
|||||||||||
How can you work out if you are having an early or late fruiting body maturity season?
A mathematical model of yellow spot ascospore maturation has been developed and tested for accuracy of prediction using the quantitative data obtained from these epidemiology trials. The ascospore maturity model uses actual weather data obtained from weather stations and epidemiological parameters from the literature to predict when the fruiting bodies of the yellow spot fungus will contain ascospores that are sufficiently mature to be released and initiate yellow spot infection. We have compared the observed ascospore maturation dates (actual stubble samples) with the predicted ascospore maturation dates (model prediction) to test the accuracy of this model for all of the locations and years shown in Table 2.
Within the ‘National pathogen management modelling and delivery of decision support’ project, a yellow spot fungicide decision support tool is being developed that will provide the economic potential for a wheat crop based on location, variety and cost of fungicide application. This yellow spot decision support tool will be linked to the yellow spot ascospore model described here.
Conclusions
Ascospores produced on the previous season’s wheat stubble are the source of primary inoculum that initiates yellow spot outbreaks in WA. Moisture determines when the fruiting bodies form and temperature determines the rate at which the ascospores mature within the fruiting bodies. The wetter, cooler autumn conditions in the southern coastal cropping area of WA (Albany and Esperance) favour earlier spore maturation compared with the central and northern cropping areas. When ascospore release coincides with the presence of a susceptible crop and favourable weather, then disease development is most likely.
It was originally thought that yellow spot was not a consistently yield limiting disease of wheat in the southern costal cropping area because it was “too cold”. These epidemiology trials have shown that in these areas a high proportion of the spores are released from the fruiting bodies before wheat crops are sown or have emerged and hence the primary infection opportunities are limited; this combined with cooler temperatures which do not favour secondary spread, can limit the impact of yellow spot.
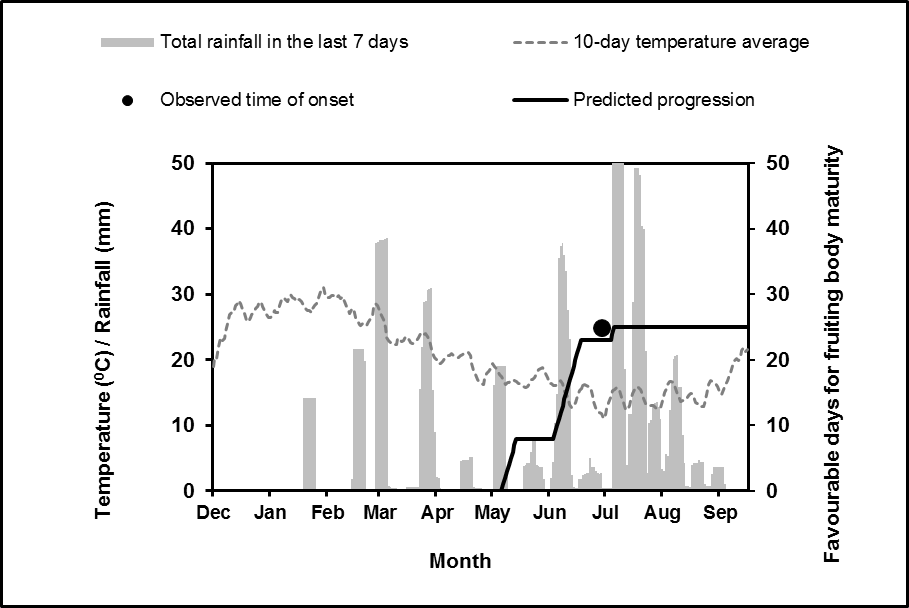
Figure 2. An example of the yellow spot spore maturity model output for Eradu in 2015. The observed time of onset and the predicted time of onset are within seven days of each other. Note the predicted progression ‘flat lines’ after the onset of maturation in the spore maturity model as this is the point at which this model will interface with the secondary spread model.
The temperature and moisture conditions in the central and northern cropping areas of WA usually favour yellow spot ascospore development after the wheat crop has been sown. In these areas spore release from stubble coincides with the growth of the crop and multiple primary infection opportunities occur. This, coupled with warmer conditions which drive secondary spread provides an explanation as to why yellow spot is often more prevalent in young crops in the northern agricultural regions compared with the southern agricultural regions.
Some years early season fungicide treatments (in-furrow or tillering/Z31 sprays) reduce the development of yellow spot while in other years, these early fungicide applications show little to no efficacy in reducing the development of this disease. Knowing the primary infection window has helped understand timing of disease development in continuous wheat crops and clarified some of the factors surrounding why fungicide trials can give variable results between seasons. In years when yellow spot maturation occurs early in the season, early fungicide applications may reduce the number of successful primary infections and potentially reduce the level of disease which develops during the season. In years when yellow spot maturation occurs later in the season these early fungicide applications are ‘ineffective’ as they are providing protection before the window of infection has opened.
The ascospore maturity model will help growers and agronomists predict the timing of yellow spot spore maturity for different locations. This information should assist in helping make decisions about which seasons might be suitable to use early fungicide treatments versus seasons in which only later applications should be considered.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through the support of the GRDC, the authors would like to thank them and the GRDC for their continued support. We would also like to thank Tess Humphreys, Anne Smith, Kith Jayasena, Lucy Debrincat, Andrea Hills (DAFWA); Rohan Kimber (SARDI) and Mel Cook (AgricultureVictoria) for assistance with stubble collection and processing and Geoff Thomas for reviewing this paper.
Contact details
Jean GallowayDepartment of Agriculture and Food Western Australia
PO Box 483, Northam, WA 6401
Ph: 08 9690 2172
Fx: 08 9622 1902
Email: jean.galloway@agric.wa.gov.au
GRDC Project Code: DAW00228,
Was this page helpful?
YOUR FEEDBACK
