Insect pest management update: Canola aphids, Russian wheat aphid, and Rutherglen bug (Pallamallawa)
Author: Melina Miles, Adam Quade, Paul Grundy & Richard Lloyd | Date: 18 Jul 2017
Queensland Department of Agriculture and Fisheries, Toowoomba
Take home messages
- Canola is most susceptible to yield and oil loss with infestations of aphids from bolting. This is earlier than previously thought, but still requires at least 10 to 14 days of infestation to significantly impact on the crop.
- Russian wheat aphid (RWA) has not yet been detected north of Rankin Springs in the Riverina district of NSW. Vigilance is required to ensure early detection of any RWA outbreaks in the northern region this winter.
- Rutherglen bug (RGB) will impact on the yield and viability of sorghum. Thresholds are available; early grain fill is the most susceptible crop stage. At extremely high densities, RGB control with insecticides can be challenging. Be aware that some commonly used products have limited residual efficacy and may not provide sufficient control to prevent crop loss.
- Research to commence on options for minimising the movement of RGB from canola stubble into neighbouring summer crop.
Aphid thresholds in canola
Over the past 3 years, DAF entomologist Paul Grundy has been undertaking research to determine the susceptibility of canola to cabbage aphid infestations during flowering and seed set. The initial work, simulating late infestations on the terminals of filling/maturing racemes, showed that canola was compensating for terminal damage and/or the affected terminal pods were not contributing significantly to yield. Recent trials (2016) have shown that cutting stimulates atypical compensation, and that bagging better simulates aphid impact, which is to prevent normal development of racemes (Table 1).
Cutting to simulate aphid damage | Bagging racemes to simulate aphid damage | ||
|---|---|---|---|
Treatment | Yield (t/ha) | Treatment | Yield (g/plot) |
Control | 2.07 a | Control (unbagged) | 1293 a |
10% of terminals removed | 1.93 a | 1. Raceme covered first flower | 988 b |
50% of terminal removed | 1.98 a | 2. Raceme covered first flower +7 days | 989 b |
90% of terminal removed | 2.01 a | 3. Raceme covered first flower +14 days | 949 b |
In 2014, trials at 8 locations on the Liverpool Plain produced similar results, with significant yield loss only occurring when the plant was forced to replace all the reproductive structures removed at early flowering (Figure 1).
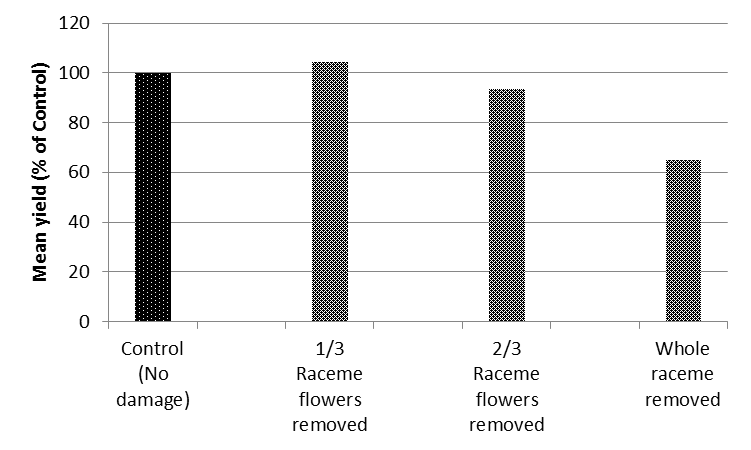
Figure 1. Aggregate treatment yields across 8 sites near Spring Ridge NSW, 2014, expressed as a percentage of the control.
The conclusion of this research was that the widely used best-bet threshold of 10% to 20% of racemes infested during late flowering-pod set, is probably conservative and could be revised up. The compensatory ability of the crop allows time for biological control by aphid parasitoids, lacewings, hoverflies and ladybeetles to take place during the first weeks of flowering without significant crop loss. If natural enemies were ineffective in supressing aphid populations, spraying on increasing level (20% to 25% of racemes infested) would be unlikely to result in irrecoverable crop damage. Very late infestations of aphids have very limited damage potential as the associated disruption mainly effects flowers/pods that are unlikely to contribute to final yield.
In 2016, replicated trials at Wellcamp near Toowoomba were undertaken with infestations of aphids, rather than simulated damage. This approach provided an opportunity to examine both the impact of aphids on crop growth and oil content. Conditions for crop compensation were excellent with approximately 300 mm of rain between June and September. Plots were infested with aphids prior to bolting to get racemes infested as early as possible. In the early sown trial (sown 9 April, infested 16 July) the infestations did not establish before bolting, but were well established through flowering. Infestation in the later sowing (sown 17 May, infested 10 August) resulted in successful early infestation so flowering racemes were heavily infested from early flowering. Infestations resulted in every plant having some aphids established in each treated plot. Greater impacts on both the yield and oil content were observed in the later sown trial (Figure 2).
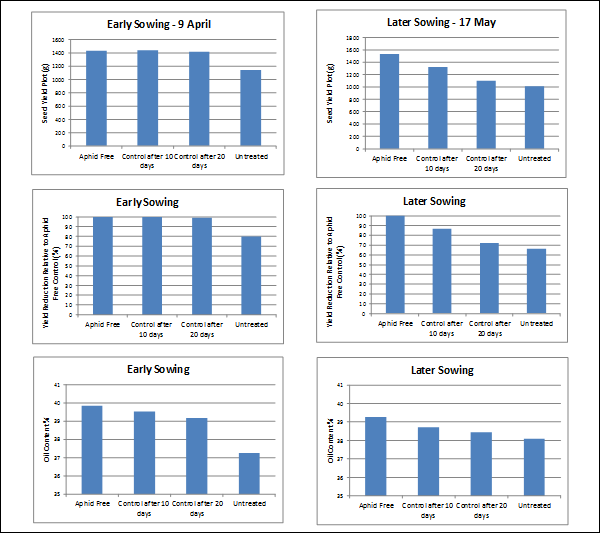
Figure 2. Very early infestation of flowering racemes with cabbage aphid for a 10 day period can reduce both yield and oil content. Result of replicated trials, Wellcamp 2016.
What the 2016 trial results mean for canola aphid management recommendations
The series of trials over the past 3 years have indicated that infestations in early flowering result in greater impacts than later infestations, and this work reinforces this.
The most susceptible period is the 2 to 4 weeks in which the plant is bolting and starting to flower, this is when crop monitoring for aphids should start – and which is probably earlier than it is done currently. To simplify sampling, assess aphid infestation in terms of % plants infested, rather than % racemes infested.
As significant yield and oil content declines did not occur with infestations of 10-14 days, there is time to assess the rate at which the aphid infestation is growing, and the potential impact of natural enemies and weather on infestations.
At this stage, a threshold of 20% of plants infested is proposed. Further work is required to validate this approach.
Russian Wheat Aphid (RWA) as a pest in the northern grains region
RWA is considered a high priority pest by the grains industry because of its potential to cause significant yield losses in wheat and barley if not well managed. Triticale and rye are also susceptible to crop loss, but oats are considered relatively tolerant.
The RWA is more damaging to cereals that the aphid species we already have in Australia. We don’t yet know how susceptible Australian varieties are to RWA. GRDC is investing in the development of resistant cultivars for Australia. In the meantime, international experience with RWA has been that in seasons following outbreaks, yield losses are generally lower as growers are better equipped to detect and manage infestations, and natural enemies establish and contribute to the suppression of populations. In South Australia and Victoria in 2016, infested crops that were treated to control RWA, recovered to grow and yield normally.
It is inevitable that RWA will establish in the northern grains region, but we don’t know when we will start to see it in crops. Given how widely distributed RWA is in SA and Victoria, it seems likely that RWA has been in Australia for some time prior to the 2016 outbreak. An outbreak is most likely in the event of favourable conditions for RWA populations to both oversummer, build up in winter and move into early crops in autumn. The environmental conditions that favoured the outbreak in the southern region were a wet summer (summer hosts), a warm autumn and early sowing of winter cereals (favour rapid population growth and movement from weed hosts to crops). In addition to crop hosts, non-crop and pasture grass species in the genera Poa, Bromus, Hordeum, Lolium, and Phalaris may also host RWA. It remains to be seen whether RWA over-summer on grass hosts or crops (e.g. sorghum, maize, millet, canary) in the cropping landscape.
In the 2017 season, it is important that crops are monitored more frequently than they might usually be to ensure early detection of RWA, should it occur.
Sample for RWA in the same way you would sample for other cereal aphids. Concentrate on the margins of the field and in areas of the paddock that are stressed (e.g. dry, wet, root disease). Look for both symptoms (see description below) and the presence of aphids.
The following thresholds are recommended:
- Emergence to tillering - 20 RWA per plant; and
- Tillering onwards (Z30-59) – 10 aphids per tiller.
If control of RWA is warranted, there is a current Emergency Use Permit APVMA PER82792 is for chlorpyrifos and pirimicarb. Pirimicarb will kill aphids, but not the beneficial insects in the crop. If possible, use this option first to preserve beneficials which may then suppress further outbreaks.
Leaf symptoms caused by RWA infestations – what to look for in the field
RWA induce striking symptoms in wheat and barley, unlike the oat and corn aphid which produce no obvious symptoms. Within a week or so of being infested with RWA, plants will start to exhibit symptoms. Plant damage is in response to direct aphid feeding, so only the leaves and/or tillers infested show symptoms.

Figure 3. Symptoms of Russian wheat aphid
Some of these symptoms are similar to those caused by wheat streak mosaic virus (WSMV) and phenoxy herbicide damage in cereals –close examination of symptomatic plants to determine the presence of RWA is recommended.
Distinguishing RWA in the field
Use a hand lens to check for key features (Figure 4), specifically for the absence of siphunculi and the double tail (cauda), characteristic of RWA.
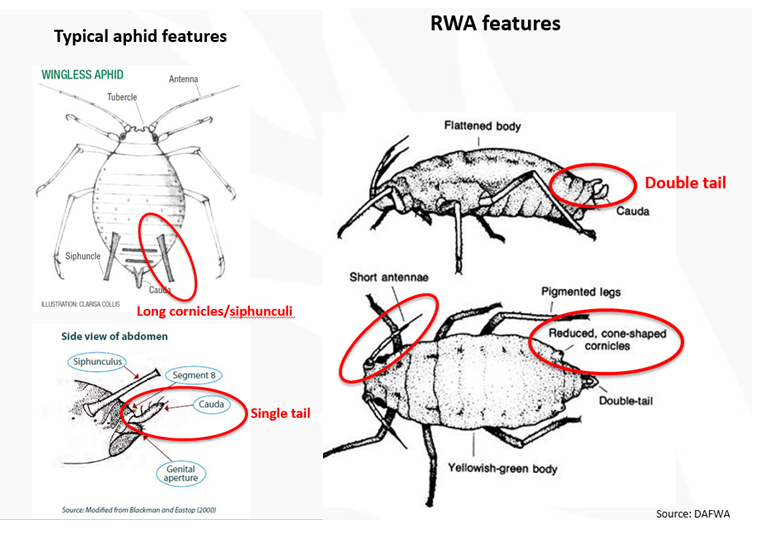
Figure 4. Features to look for in a Russian wheat aphid
Rutherglen bug (RGB)
RGB movement from canola stubble – damage to establishing summer crop
To date little, if any, research has been focused on this phenomenon. QDAF entomologists have done some preliminary work, which has provided a little insight. This issue is a high priority research area for 2017.
RGB nymphs (all developmental stages) and adults typically move en masse from canola stubble as it dries down in October-November. The movement out of the canola stubble is in all directions and appears to be an exodus rather than directed movement towards summer crops. In southern Australia, the same movement occurs and is of little consequence as there are no summer crops. Similarly in central NSW, the benefit that RGB provide by feeding on and killing canola regrowth, volunteers and weeds is appreciated by growers as a benefit.
RGB moving into summer crop will congregate on seedlings and the sheer impact of feeding results in plant dehydration and death. Repeated spraying of crop edges slows the movement of the RGB further into the summer crop, but does not prevent the death of plants in rows closest to the canola. The movement from the canola stubble can continue for weeks.
The number of RGB moving has also made barrier strips, ploughed strips and water-filled channels ineffective in stopping the movement. The absence of effective, long residual products that can be applied to bare earth between canola stubble and summer crops is a major constraint in having a simple management option.
There are 3 key approaches being considered for research to resolve this problem.
- Preventing the build-up of RGB populations in canola stubble. Understanding the ecology and behaviour of RGB in the canola crop to determine when eggs are being deposited (in the soil). One of the key questions is around the potential impact of RGB to canola. Knowing what impact RGB has on other crops (sunflower and sorghum) during grain fill, it is possible that there may be benefits to the canola crop from controlling RGB during flowering-harvest.
- Effective options to minimise the movement of RGB between canola stubble and summer crop. In the absence of effective bare-earth residual options, we are considering the potential of trap crops to slow, and possibly stop the movement of RGB. Neonicotinoid seed treatments are essentially a way to deliver a highly effective residual insecticide to RGB. We expect that it may be possible to have migrating RGB populations stop, feed and then die in a trap crop planted between the canola stubble and the susceptible summer crop. The trap crop would be maintained only for the period during which RGB were moving. This approach is completely novel, and will be trialled for the first time in the spring of 2017.
- Reducing the RGB populations within canola fields. Evaluating options to reduce the size of the population moving out of the stubble through the application of less disruptive options within the canola field. For example, the use of diatomaceous earth, biopesticides, spray oils, swathing insecticide (rather than complete coverage) are options to be trialled for reducing the impact of whole-field treatment with broad-spectrum insecticides as is being done currently.
RGB in sorghum
In the spring-summer of 2016-17, the northern grains region experienced a major outbreak of Rutherglen bug (RGB, Nysius vinitor).
Until 2007, RGB was not considered a major pest of sorghum although it has been recognised as a major pest of sunflowers for a long time. Possibly, RGB were controlled in sorghum when crops were treated for midge. However, in 2007, extremely high infestations of RGB in early sorghum crops resulted in damage; heads with low percentage of seed set. In 2007-08, QDAF entomologists undertook research to understand the impact of RGB in sorghum.
In 2016-17, RGB populations were extremely high, and they persisted in crops for weeks. At the same time, heatwave conditions were affecting sorghum crops. In combination, the RGB and the heat resulted in many sorghum crops with low yields and poor quality. In this paper, we describe RGB damage, so it can be distinguished from heat damage.
As if this wasn’t enough, control of RGB proved to be a challenge for some. Questions were asked about the efficacy of products and the possibility that RGB were resistant to some insecticides. In response to the challenges with effective control of RGB in 2016-17, QDAF entomologists screened a large number of insecticides to evaluate both contact and residual efficacy. The results of this work, as it relates to RGB control in sorghum, is presented here.
RGB damage and thresholds in sorghum
Rutherglen bug females need to feed on developing seed before they can start to produce eggs. Consequently, damage to sorghum at the susceptible crop stages (flowering – soft dough) is caused by adults only. Nymphs are generally present in sorghum crops as the grain starts to colour.
Adult feeding results in seed that does not fill, or seed that is discoloured (dark red) with black feeding spots (Figure 5A, B). The seed is not typically ‘beaked’ as occurs with head stress (Figure 5C).
The major impact of RGB feeding is poor seed set and low grain weights. RGB are particularly damaging in seed production where their feeding also results in greatly reduced germination rates of resulting seed.

Figure 5. Typical RGB damage to sorghum (A) poor seed set at the top of the panicle similar in appearance to midge damage, (B) reddening and spotting on the seed. (C) heat-damaged sorghum seed, note the reddening, but absence of black feeding marks.
Based on trial work conducted in 2007-08, the proposed economic thresholds for RGB in sorghum are presented in Table 2. The thresholds reflect the susceptibility of the grain whilst filling, and the lack of impact once the grain is filled and reaches physiological maturity. These results show that treating large populations of RGB in sorghum close to harvest has no benefit in terms of yield, but may reduce the likelihood of problems with large populations at harvest (blockages, moisture, live insect contamination).
Stage of development | Threshold (RGB per head) |
|---|---|
Flowering and milky dough | 20-25 |
Soft dough | 30-50 |
Physiological maturity (black layer) to harvest | No impact on yield* |
*large populations of RGB in the crop at harvest may be a contaminant and occasionally cause blockages because of the increased moisture from the RGB in the grain. | |
Chemical control of RGB
Screening trials (laboratory based Potter Tower bioassay) showed that alphacypermethrin, deltamethrin and dimethoate were highly effective in killing RGB when sprayed directly onto adults. Please note – these products are currently registered for control of RGB in other crops, but not in sorghum.
However, when RGB populations are large it is unlikely that commercial application will get good direct contact on all individuals. This is likely to be more problematic when large populations of adults and nymphs are targeted. Therefore, the residual efficacy of the insecticides becomes important (how toxic they are when RGB make contact with dry residues on leaves/stems/heads). Again the same suite of products was tested in a lab test with RGB introduced to leaves that had been treated with each of the products at 3 and 7 days after spraying. The number of RGB adults alive/dead was assessed after 24 hours of exposure to the treated leaves. Results showed the residual efficacy of the treatments varies, with rapid deterioration of alphacypermethrin after 3 days, and further loss of efficacy of alphacypermethrin and low rate dimethoate after 7 days. These results support the anecdotal observations of many agronomists and growers in the field this past summer.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
Contact details
Melina Miles
Queensland Department of Agriculture and Fisheries
PO Box 102, Toowoomba. Qld 4350
Ph: 0407 113 306
Email: melina.miles@daf.qld.gov.au
GRDC Project Code: DAQ00196,
Was this page helpful?
YOUR FEEDBACK
