Wheat and barley disease update for Victoria - 2017
Author: Grant Hollaway, Mark McLean (Agriculture Victoria, Horsham) and Andrew Milgate ( NSW DPI, Wagga Wagga) | Date: 23 Aug 2017
Take home messages
- Cereal diseases will need active management during 2017 following carry-over of high levels of inoculum on stubble and the green bridge after the wet spring of 2016.
- Consult a current disease guide to review your varieties’ resistance/susceptibility to the important diseases in the region and plan accordingly.
- There is resistance to fungicides in some important wheat diseases which highlights the need to rotate fungicide actives within crops and to implement integrated disease management strategies.
- Proactive disease management strategies are effective for disease control and provide economic return in seasons favourable for disease.
Background
During 2016 the wettest spring in 25 years was experienced which created ideal conditions for crop diseases. Without disease control, yield losses of 20% or more were common in susceptible varieties. These ideal conditions have resulted in higher than usual levels of stubble borne inoculum and combined with the green bridge (volunteer cereals growing over summer/autumn) higher levels of rust inoculum for infection of 2017 crops.
Cereal disease management in 2017
With the high inoculum loads present this year, it is important that an active approach to disease control is adopted. Disease management plans need to consider varietal ratings (consult a current disease guide) and inoculum loads within a paddock (i.e. stubble and soil borne diseases) and the district (i.e. the green bridge). Develop a fungicide strategy for each crop based on the identified risks, as cost effective control can be achieved when a proactive approach is used.
Leaf rust in wheat
Two new strains of wheat leaf rust were detected during 2014 and 2016, and are now important in eastern Australia. The effect of these new strains on wheat cultivars are shown in the current Victorian Cereal Disease Guide. Many cultivars are now one rating more susceptible than before. Several cultivars (including Beaufort, Corack, Derrimut, Mace, SQP Revenue and Wallup) are now two or more rating levels more susceptible (Table 1). Should conditions be suitable for leaf rust development this year, growers will need to be vigilant with its control, noting that leaf rust becomes important later in the season (after ear emergence).
Cultivar | Wheat Leaf Rust Rating Changes | |
|---|---|---|
2014 | 2017A | |
SQP Revenue | Resistant® | Very susceptible (VS) |
Beaufort, Derrimut, Livingston | R | MS- susceptible (S) |
Lincoln | Moderately resistant (MR) | S |
Corack, Wallup | Moderately susceptible (MS) | SVS |
Bolac, Chara | MS | S |
A Always consult a current disease guide.
Septoria tritici blotch
Septoria tritici blotch (STB) has become the most important disease of wheat crops in Victoria’s high rainfall zone. Its management is becoming increasingly difficult with resistance to fungicides becoming common. Integrated disease management strategies need to be implemented along with strategies to delay further fungicide resistance development.
Stubble management
Since STB is primarily a stubble borne disease, both crop rotation and stubble management can contribute to control. In most instances, a one-year break from wheat is effective in reducing early disease occurrence. Do not sow wheat on wheat. Any tillage that reduces stubble on the surface (such as burial, burning or grazing) can reduce inoculum levels — but these practices need to be balanced with the risk of soil erosion. Stubble management will not reduce disease caused by spores blown from other paddocks.
Variety selection
Avoiding susceptible and very susceptible varieties (ratings of S, SVS or VS) is an effective strategy to reduce in-crop disease and historically has provided long term disease control. Since STB is pathogenically diverse (with different strains able to attack different varieties), and resistance breakdown known to occur, it’s important to consult a current disease guide. The GRDC and Agriculture Victoria support screening of current varieties, National Variety Trials (NVT) entries and pre-breeding wheat lines for STB resistance. The ratings are published in the annual cereal disease guide.
Fungicides
Fungicides can contribute to STB control, especially during wet seasons. In early sown susceptible varieties, where infection is established during autumn, an early fungicide application at Z31-32 can be effective at suppression of early disease and protect emerging leaves. Another fungicide application at full flag leaf (Z39) can protect the upper canopy.
STB is developing resistance to triazole fungicides. Surveys and studies by NSW DPI have shown reduced effectiveness of triazole fungicides in southern Australia. A mutation known at Cyp51 isoform 11, that was first identified in Tasmania has now been detected in the Victorian HRZ and is expected to become dominant in Victoria in the next few years. This means growers need to choose triazole fungicides carefully for disease control. Product formulations relying only on flutriafol, tebuconazole, propiconazole and cyproconazole will not provide full protection against STB Cyp51 isoform 11.NSW DPI research has demonstrated that products containing fluquinconazole has higher levels of efficacy to all current isoforms present in Australia. In areas of high disease pressure growers should consider the use of fluquinconazole to reduce early season disease pressure.
No changes have been identified to the resistance status to strobilurins in Australia. This mode of action is still effective and can be part of a fungicide management strategy for STB. It is worth noting that Australia is one of the few places in the world where strobilurins are still effective, and therefore, users should be careful in managing this class of fungicides for the future. The strobilurins are at high risk of selecting for resistance when used repeatedly within a growing season.
No changes have been identified to the resistance status to succinate dehydrogenase inhibitors (SDHI) in Australia. With SDHI products becoming available in Australia it is worth noting that STB has developed resistance to this mode of action in the UKɸ. This mode of action requires proactive management in order to sustain its useful life. Avoid applying an SDHI more than once in a season.
ɸCurrently no SHDI products registered for control of STB in Australia.
Since STB is developing resistance to fungicides, it is critical that strategies are used which reduce the likelihood of further resistance developing, and hence extend the useful life of currently available fungicides:
- Do not use the same triazole active ingredient more than once in a season. Do not use a strobilurin or SDHI more than once in a season.
- Aim for early control of disease. STB spreads up the leaf layers of the canopy through rain splash and direct leaf contact. Reducing the disease in the lower canopy slows the upward movement of disease and ultimately the leaf area lost.
- Follow label instructions at all times.
Recent studies by NSW DPI have shown that some triazoles (triadimefon, tebuconazole, propiconazole, flutriafol and cyproconazole) have become less effective for STB control. Other triazoles (epoxiconazole and fluquinconazole) are still useful for disease control, even though they have reduced efficacy. The strobilurins and SDHI groups are still effective.
In addition to mixing or rotation of fungicide actives, an integrated approach to disease control is required to reduce the likelihood of further fungicide resistance developing, by keeping inoculum loads low. The approach will include crop rotation, avoiding susceptible cultivars and delayed sowing.
Powdery mildew in wheat
Powdery mildew in wheat has increased in importance in some growing areas around Australia as some new wheat varieties being grown are susceptible. The Centre for Crop and Disease Management (CCDM, Curtin University) has identified resistance to strobilurin fungicides (resistance group 11) in wheat powdery mildew isolates from Victoria and Tasmania during 2016. The CCDM, based on their recent testing, are also concerned that reduced effectiveness of the triazole fungicides (resistance group 3) is becoming increasingly likely.
Growers are advised not to apply the same active to a crop more than once per season and to implement an integrated disease management strategy that minimises disease development.
Pseudo black chaff in wheat
During 2016 there were many reports of a black discoloration on the stems and/or glumes of some wheat varieties. This darkening is a melanisation of the tissue that is known as pseudo black chaff or false black chaff. It occurs sporadically from season to season and generally is more noticeable in warm humid conditions as the crop matures. It is not detrimental to yield and there is no control.
It is a physiological condition caused by the deposition of melanoid pigments and is associated with the adult plant (APR) stem rust resistance gene Sr2. This resistance gene is the most widely deployed resistance gene globally and has been used in plant breeding for 100 years. Common Victorian wheat varieties with the Sr2 resistance gene, and therefore, varieties prone to expression of pseudo black chaff include: Mace, Derrimut, Cobra, Cosmick, Corack, Emu Rock, Livingston, Mansfield, Mitch, Scepter, Wallup and others.
Scald in barley
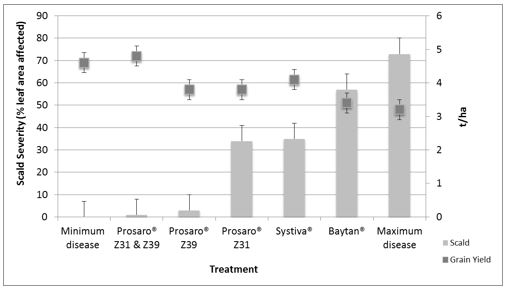
Severe scald infection developed in many barley crops during the 2016 season due to sustained cool, wet weather conditions. There will be significant inoculum present this year and it is important to control scald early, as it develops rapidly in the presence of favourable conditions and can cause severe losses in susceptible varieties. Experiments at Horsham showed severe scald can cause up to 33% (1.4t/ha) grain yield loss (Figure 1) and significant reductions in grain plumpness.
Scald is best managed with a dual foliar fungicide application approach consisting of applications at Z31 and Z39. Systiva®ɸ was very effective at suppressing scald early in the growing season but is less effective after tillering with a follow up foliar fungicide often required to minimise losses if favourable conditions occur. Baytan® was also found to provide some suppression during the seedling stages of crop development, but should not be relied on for complete scald control.
ɸSystiva is a registered seed treatment product.
Figure 1. Scald severity (% leaf area affected) and grain yield of susceptible barley variety, Yagan in response to different fungicide treatments at Wonwondah, during 2013.
Spot form of net blotch (SFNB) in barley
Spot form of net blotch was severe in susceptible varieties planted into infected stubble during 2016. Grain yield loss of up to 2.5t/ha (35%) was recorded in an experiment at Horsham (Table 2) highlighting the importance of disease control. Losses were reduced by avoiding very susceptible varieties. Yield loss varied from 18 to 35% in very susceptible varieties compared to 16 to 20% in susceptible varieties. The lowest loss was 15% in the moderately susceptible variety Scope. All susceptible varieties require fungicide management where disease pressure is severe.
Grain yield loss | |||
|---|---|---|---|
Variety | SFNB severity (% leaf area affected) Z71-85 | t/ha | % |
Dash (VS) | 60 | 2.5 | 35 |
Hindmarsh (VS) | 49 | 1.9 | 26 |
La Trobe (S) | 33 | 1.5 | 20 |
SY Rattler (VS) | 41 | 1.2 | 18 |
Scope (MS) | 19 | 1.1 | 15 |
Granger (S) | 29 | 1.0 | 16 |
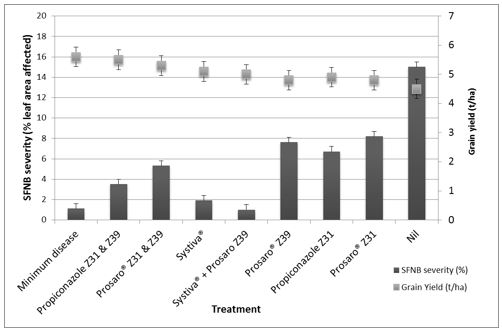
A proactive fungicide management approach can minimise losses from SFNB. A dual foliar fungicide application approach consisting of treatment at stem elongation (Z31/32) and flag emergence (Z39) has consistently provided the greatest SFNB suppression and improvements in grain yield (Figures 2 and 3) in our experiments. Seed applied Systiva® or a foliar fungicide applied at Z31/32 and again at Z39 is a good strategy as it provides sustained SFNB suppression throughout the season. Even though a single application of foliar fungicide or seed applied Systiva® can provide improvements, better control is achieved with a dual approach.
Figure 2. Spot form of net blotch severity at ripening and grain yield of Rosalind (SVS) barley in response to fungicide treatments at Curyo during 2016.
Foliar fungicide chemical choice is less important than application timing. Our experiments have shown similar SFNB suppression and grain yield when Prosaro® (150 ml/ha) and propiconazole (600 ml/haɸ) were compared (Figure 3). This indicated that both options are good for SFNB management if applied at the right time (Z31/32).
ɸ600 ml/ha is a rate used for trial purposes only as the registered rate for propiconazonle (Tilt) which is 500ml/ha).
Figure 3. Spot form of net blotch severity (%) on the top three leaves at ripening and grain yield of Rosalind barley in response to fungicide treatments at Horsham during 2016.
Net form of net blotch (NFNB) in barley
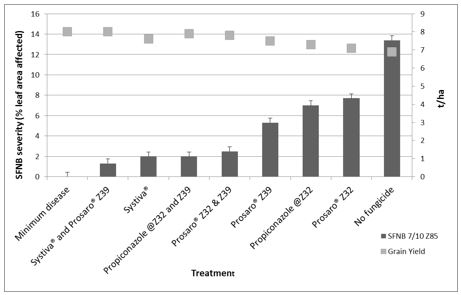
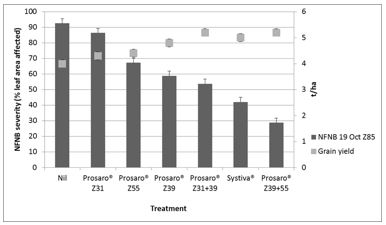
Net form of net blotch is becoming more common in Victoria with the increase in susceptible varieties such as Fairview, Oxford and Planet. Where possible avoid susceptible varieties as up to three fungicide applications may be necessary to control this disease. In an experiment at Horsham in 2016, none of the fungicide treatments provided total control due to the severity of the disease. The best management strategy consisted of dual foliar fungicide applications at either Z31 and Z39 or Z39 and Z55 (Figure 4). Systiva® was effective at suppressing NFNB during the first half of the season, but required a follow up foliar application to minimise loss. Single foliar fungicide application strategies were only partially effective. The aggressive nature of this disease means that three or more applications of fungicide may be required to minimise loss in seasons favourable for disease.
Figure 4. Net form of net blotch severity (% leaf area affected) and grain yield of a very susceptible barley breeding line, VB9613 in response to fungicide treatments at Horsham, during 2016.
Crown rot
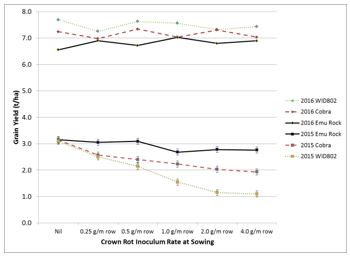
Losses from crown rot were minimal during 2016 as good spring rainfall does not favour this disease. Crown rot is favoured by close cereal rotations, stubble retention practices and seasons with below average spring rainfall. Crown rot losses are usually about 5% each season, but can be greater than 20% where abundant inoculum is present. Yield losses of up to 60% occurred in 2015 (dry season), but not in 2016 (wet season) with increasing levels of crown rot inoculum at planting (Figure 5).
Figure 5. Effect of increasing crown rot inoculum levels at sowing on the grain yield (t/ha) of durum wheat (WID802) and bread wheat (Emu Rock and Cobra) grown at Dooen during 2015 with a dry spring (p<0.001, LSD = 0.23) and 2016 with a wet spring (n.s). Note: study conducted in collaboration with Simpfendorfer et al., NSW DPI (DAW00245).
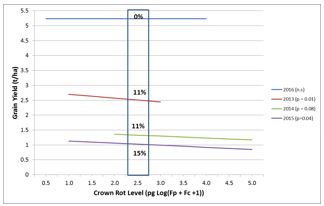
Crown rot must be managed prior to sowing as there are no in-crop controls available. Inspection of previous cereals for crown rot symptoms (browning on stem bases) provides an indication of risk from crown rot. A PreDicta B soil test is a good option to identify crown rot risk in paddocks before planting. Four years of field trials demonstrated the relationship between pre-planting crown rot inoculum levels and grain yield in bread wheat (Figure 6). This shows the usefulness of a PreDicta B test in identifying crown rot risk and shows that crown rot risk is less in seasons with a wet spring.
Figure 6. Grain yield of bread wheat decreases in seasons with dry springs (2013, 2014 and 2015, bottom three lines), but remains unchanged in a season with a wet spring (2016, top line) as levels of crown rot (Fusarium pseudograminearum and F. culmorum) increase, as determined using the PreDicta B test before planting in four field experiments at Horsham, 2013 to 2016. The box shows the percentage yield loss at the medium to high PreDicta B risk threshold.
In paddocks with medium to low crown rot levels, yield losses can be minimised by avoiding durum wheat and growing barley in preference to bread wheat. Even though barley is a good option for reducing yield loss from crown rot, it will increase inoculum levels for the next crop.
In paddocks with high levels of crown rot it is best to avoid cereals. Cereals increase inoculum levels while broadleaf crops and fallow decrease the inoculum levels of crown rot. In general, a two year break from cereals is required to reduce medium to high inoculum levels to a low level.
Take-all
It is likely that take-all levels will be higher in 2017 than in recent years. There were increased reports of take-all causing white heads in wheat crops during 2016 and its build up would have been further favoured by the wet spring.
Following cereal paddocks, or where grasses have not been controlled, a PreDicta B test before planting cereals can identify paddocks at take-all risk. In paddocks with a take-all risk, sowing non-cereal crops can be considered. Seed or fertiliser treatments can be used if cereals are to be grown in medium risk cases. Take-all can be controlled with a one year break from cereals and grasses.
Bunts and smuts
Seed treatments provide cheap and effective control of bunt and smut diseases. Seed should be treated every year as bunt and smut can increase rapidly, resulting in unsaleable grain. Good coverage of seed is essential and clean seed should be sourced if a seed lot is infected. Fertiliser treatments do not control bunt and smuts, so seed treatments are still required.
Low levels of loose smut were observed in barley crops during 2014 to 2016. La Trobe, Hindmarsh and Spartacus CL appear to be more susceptible than other varieties and some seed treatments have not provided 100% control. Growers need to ensure that an effective seed treatment is used and good coverage is achieved. Research by Hugh Wallwork (SARDI) has shown that seed treatments that contain only triadimenolɸa provide only 50% control of loose smut in Hindmarsh. Products containing flutriafol and tebuconazole, as well as only a low rate of Rancona® Dimension (80 mL) also allow some infection to persist in crops. Effective control is provided by products containing carboxin and the new SDHI fungicides, Evergol® Prime and Vibrance®ɸb, however, where seed is known to be infected then the higher rates listed on the product labels for the control of rhizoctonia control are recommended. The higher label rates of Rancona® Dimension should also provide effective controlɸc.
ɸaCurrently there are no products registered with only triadimenol for the treatment of smut; ɸbGrowers when using products commercially must adhere to label rates, Vibrance label rates for smut is 90-180 mL/100kg seed and for rhizoctonia is 180-360mL/100kg seed; ɸcRancona Dimension label rate for use against smut is 80mL/100kg and a higher rate of 320mL/100kg for use against rhizoctonia.
Conclusion
Inoculum levels will be higher than normal for cereal diseases in the 2017 season following high disease levels during 2016. It is important that plans are developed to effectively manage wheat and barley diseases this season.
Useful resources
CropPro website - Identification and Management of Field Crop Diseases in Victoria - updated 2015
eXtensionAUS: search for 'updates on seasonal issues'
Centre for Crop and Disease Management - Sampling Report Annual Review 2016
AgVic website - Current Victorian cereal disease guide
AgVic website - Bunts and Smuts of Cereals
AgVic website - Septoria Tritici Blotch of Wheat
Submitting Septoria samples for fungicide resistance screening
The more samples of STB received the better informed growers are about the status of fungicide resistance.
To collect and submit samples please follow these instructions:
- Sample collection: Collect up to 20 leaves in total by walking in two parallel transects 10m apart collecting leaves which have STB lesions every 10m.
- Place the leaves in a paper bag and label with the following:
- Name, contact details, any fungicides applied to the crop and location.
- Then post to:
Dr. Andrew Milgate
WWAI, Pine Gully Rd,
Wagga Wagga, NSW, 2650
Acknowledgements
The research undertaken as part of this project is made possible from investment by the Victorian Government (Agriculture Victoria) and the GRDC. GRDC investment is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the author would like to thank them for their continued support.
The authors would also like to thank Fran Lopez Ruiz (CCDM) for contribution on wheat powdery mildew and Agriculture Victoria’s Cereal Pathology Technical Team: Graham Exell, Jordan McDonald, Tom Pritchett, Jon Baker, Laura Roden and Luise Sigel.
Contact details
Grant Hollaway
Agriculture Victoria, Private Bag 260, Horsham 3401, Victoria
03 5362 2111
grant.hollaway@ecodev.vic.gov.au
GRDC Project Code: DAV00129, DAN00175, DAV00136, DAW00245, DAS00137,
Was this page helpful?
YOUR FEEDBACK