Best options for optimal performance from rhizobial inoculants
Take home messages
- Peat slurry inoculants applied to seed perform consistently well; other formulations can also provide good nodulation, but outcomes are dependent on the carrier and sowing conditions.
- There is little data on the survival of rhizobia in different formulations when dry sown. Some of the first data for rhizobial survival under dry sowing conditions in southern Australia is presented.
- Agrochemicals and fertilisers at sowing can affect rhizobial survival — avoid contact between these and rhizobia.
- New acid tolerant rhizobia have improved faba bean and lentil nodulation and may increase the area where these pulses can be grown.
Background
Nitrogen (N) is the major nutrient required for grain production in Australia. Pulse crops provide an inexpensive and sustainable source of fixed N that underpins high value pulse production and provides residual N for the following cereal and canola crops. N fixation is dependent on the availability and number of suitable root nodule bacteria (rhizobia) (Drew et al. 2012, Denton et al. 2013). Pulses are estimated to fix 40kg N/ha to 136kg N/ha on average (Unkovich et al. 2010), worth about $220 million each year to Australian farming systems. However, not all pulses in Australian farming systems are well nodulated and fix optimal N. One reason for this is poor rhizobial adaptation in some soils. To increase the productivity of pulses and to expand pulses beyond their current range, particularly into acid soils, rhizobia that are better adapted to these soils are required. The development of rhizobia that improve nodulation in these soils will assist the expansion of pulses into new areas.
Current farming production systems often involve sowing crops early in the season. As a consequence, pulses such as faba bean, lupin and even chickpea in some areas are being dry sown before breaking rains. Rhizobia are sensitive to desiccation and the consequences of dry sowing to rhizobia survival for different inoculant formulations are poorly understood, as there have been limited studies in Australia to understand the extent to which dry sowing affects the nodulation, N fixation and grain yield in pulses.
Although peat slurry inoculation is very effective, different inoculant types and methods of application are also available. Granular products differ greatly in their rhizobial number, granule size, initial moisture content and efficacy when sown into moist soil (Denton et al. 2009). Understanding how dry sowing impacts on performance of these different formulations and application methods is a question often asked by growers. In addition, where dual stresses occur, such as dry sowing combined with acid soils or dry sowing combined with toxic chemicals, the consequences for rhizobial survival are likely to be increased.
Nodulation can also be detrimentally affected by the agrochemicals, fertilisers, or additives that come into contact with rhizobia at sowing. Since rhizobia are living organisms, they are very susceptible to toxic chemicals, extremes of pH, desiccation, heat and high salt concentrations. The inadvertent or deliberate mixing of different compounds with rhizobia often causes rapid cell death, leading to sometimes catastrophic nodulation failure. Where this occurs there is little recourse for growers other than to re-inoculate. Better advice is required to support recommendations about the effect of additives on rhizobial survival, and therefore, to avoid failures.
Results and discussion
Do inoculant types and application methods differ in their effectiveness?
Over the last two decades, the use of granular and liquid inoculants in Australia has increased markedly (Denton et al. 2009, 2017). Although peat slurry inoculants have been used effectively over the last hundred years in Australia, and are supplied at high quality by inoculant companies, the methods of application to seed can be inconvenient, especially to large volumes of pulse seed when there are time pressures around sowing. Granular and liquid inoculant application systems also provide an opportunity to separate rhizobia from toxic chemicals, such as pickles applied to the seed coat. Peat slurry inoculants typically contain a greater number of rhizobia, than bentonite clay granules (Denton et al. 2009), and this is reflected in the relative levels of nodulation by the different formulations across multiple trial sites (Figure 1).

Figure 1. Mean nodule numbers for rhizobia inoculation using either peat slurry inoculants or with different granular inoculant treatments applied with or below the seed. Data are means of 37 replicated field experiments conducted in SA, Victoria and southern NSW. Background nodulation for un-inoculated plants was 4.3 nodules per plant (geometric mean for the 37 experiments), shown as a dashed line. Error bars are 1% least significant difference intervals; if these overlap for a pair of treatments they are not significantly different at the 1% level. Data from Denton et al. (2009).
A comparison of peat applied to seed as a slurry, with peat-based granules and peat suspension in water injected into furrows was tested in four field experiments (Denton et al. 2017). In this study, inoculation improved nodulation (data not shown), grain yield and N fixation of faba bean (Table 1). At Mininera there were no differences in grain yields of faba bean with different inoculation methods, while at Culcairn, peat slurry applied to seed, again provided the best outcome and inoculant injected as a liquid into the furrow was lower by 1t/ha (Table 1). Similarly, there were no differences in N fixation among the different inoculation methods at Mininera, while peat slurry inoculation was superior in N fixation at Culcairn (Table 1).
Table 1. Faba bean grain yield and N fixation when inoculated with peat slurry applied to seed, peat granules or peat as a liquid suspension injected into furrows. Means in a column not followed by the same letter are significantly different at the give P values.
Treatment | Mininera, Vic | Culcairn, NSW | ||
|---|---|---|---|---|
Grain yield | N fixation | Grain yield | N fixation | |
Uninoculated | 0.94 b | 17 b | 1.75 c | 36 c |
Peat slurry | 1.42 a | 63 a | 3.69 a | 316 a |
Peat granules | 1.13 ab | 44 a | 3.50 a | 130 b |
Peat as liquid | 1.37 a | 66 a | 2.70 b | 121 b |
P <0.01 | P<0.001 | P<0.001 | P<0.001 | |
Does inoculation work when pulses are dry sown?
Growers often sow faba bean, lupin, lentil and even chickpea into dry soil. However, assessment of different inoculant types under dry sowing conditions are limited in providing much general information to growers. Sowing inoculated seed into dry soil is not recommended where a legume crop is sown for the first time, as there may not be a suitable background level of rhizobia (Drew et al. 2014). On the other hand, where a legume has been used frequently and the soil is not particularly hostile to rhizobia, the risk of nodulation failure resulting from dry sowing is much reduced. Rhizobial formulations applied in furrow, such as granules or peat suspended in liquid, can be placed at a greater depth in the soil where theoretically they will have a better chance of survival, as soil moisture and temperature fluctuations will be less extreme at these depths.
Currently only two granular formulations (supplied by Alosca and Novozymes) are recommended for use when dry sowing. Field trials were conducted at two sites in 2017 assessing a range of formulations at different rates and in combination (including peat on seed); these treatments push the boundaries of current recommendations, with the aim of providing better guidelines for growers around dry sowing. In a recent field experiment at Wanilla, all inoculants improved the nodulation of faba bean relative to no inoculation (Figure 2). Novozymes granules provided the best nodulation at two sampling times and for both sowing times, and surpassed the nodulation provided by peat slurry inoculation on seed at that site (Peat) (Figure 2).
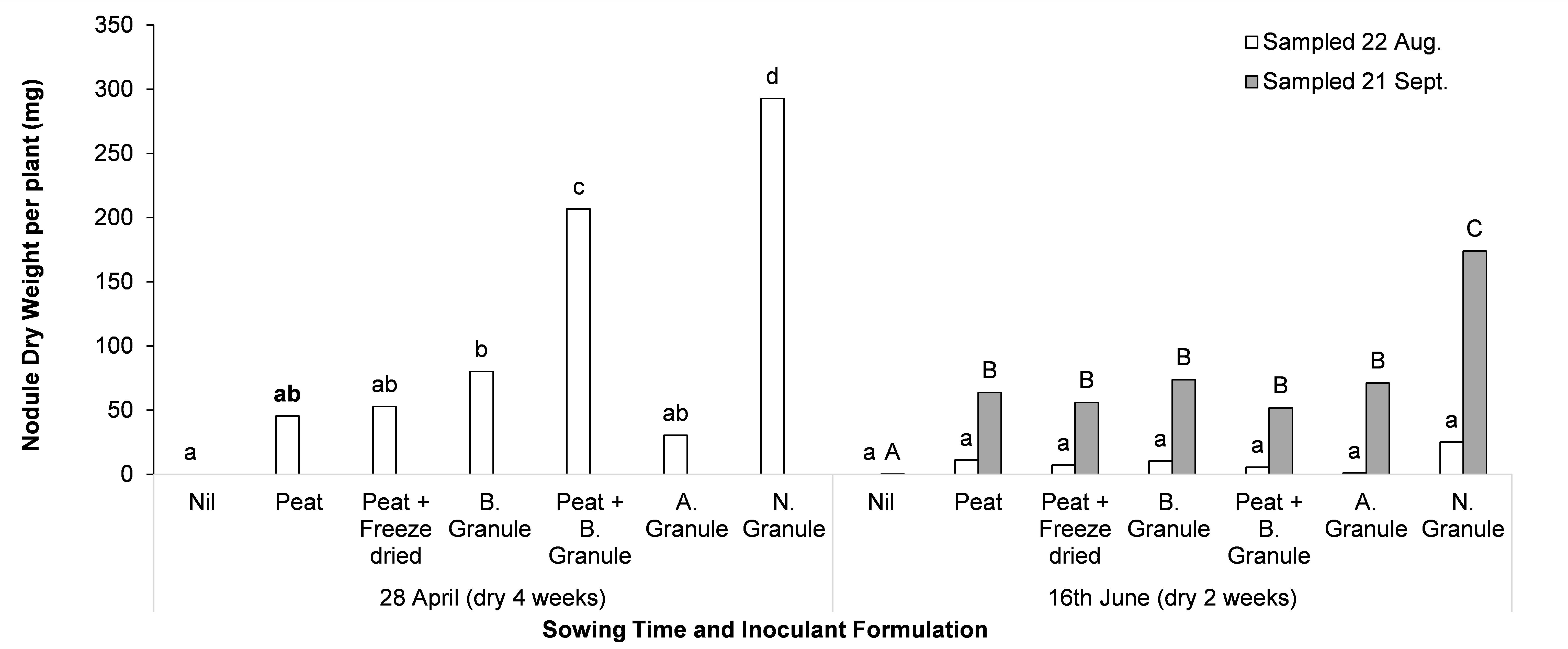
Figure 2. Nodulation of faba bean when sown dry (28 April — approximately four weeks between sowing and sufficient rainfall for germination, and 16 June — approximately two weeks between sowing and sufficient rainfall for germination) and when different inoculant applications were applied at Wanilla, SA. Nodule weight per plant was measured on the 22 of August and 21 of September. Letters that differ within a sampling time indicate significance at P<0.05. All inoculants are group F; peat slurry on seed (Peat) and BASF granules (B. Granule) were supplied by BASF and applied separately or in combination, Freeze dried inoculant (Freeze dried) was supplied by New Edge Microbials (applied with the Peat treatment), Alosca granules (A. Granule) were sourced from Landmark and Novozymes (N. Granule) were supplied by Novozymes.
In a similar study using different inoculants on lupin at Farrell Flat, mid North of SA, there were fewer differences between formulations, and granules provided similar nodulation to peat slurry. However peat slurry in combination with freeze-dried inoculant enhanced nodulation of the crop sown late into moist soil at measured at the second sampling time (Figure 3).
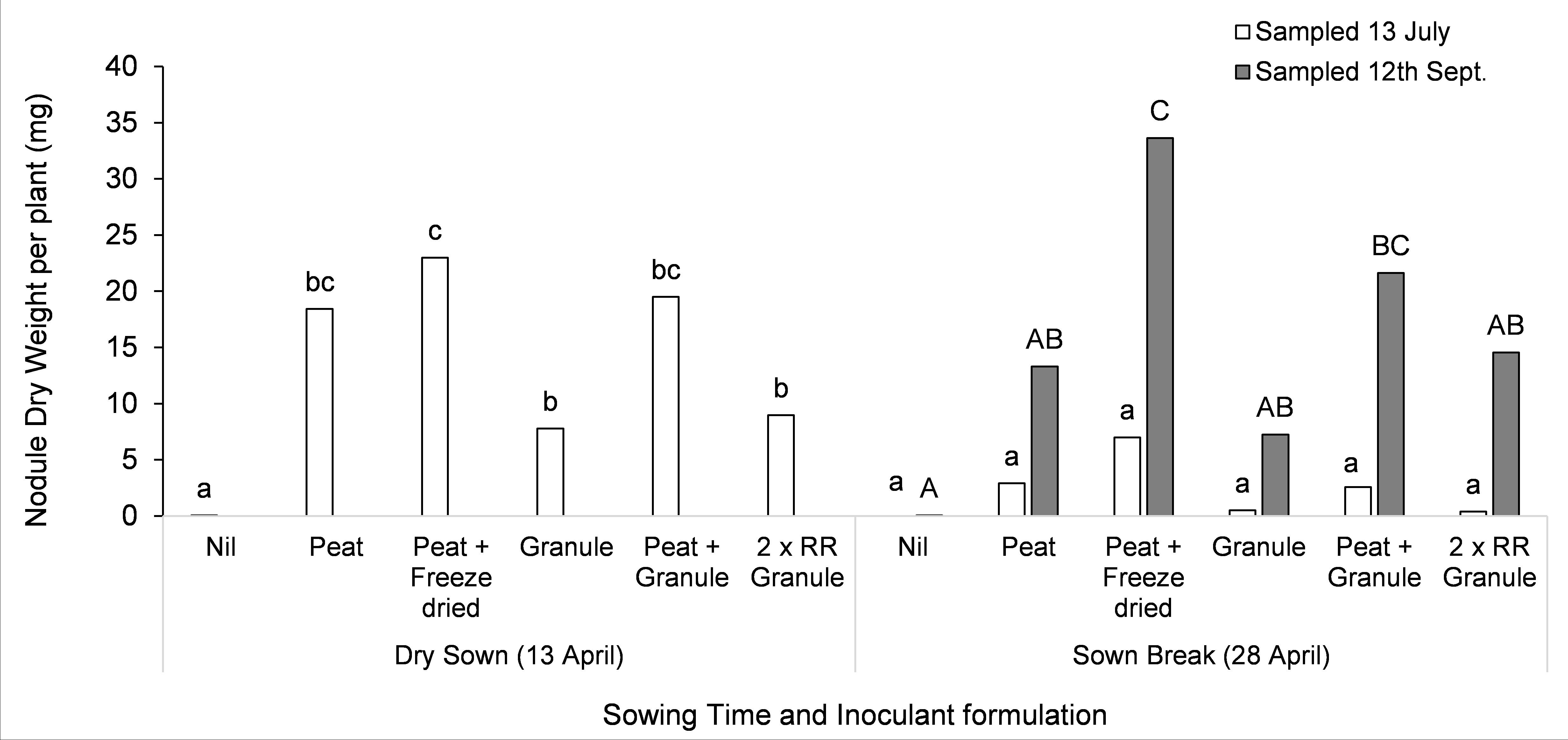
Figure 3. Nodulation of lupin sown into dry soil (13 April — seven days between sowing and sufficient rainfall for germination) or moist soil (28 April) when different inoculant applications were applied at Farrell Flat, mid North of SA. Nodule weight per plant was measured on the 13 July and 12 of September. Letters that differ within a sampling time indicate significance at P<0.05. All inoculant was group G Lupin. Peat slurry on seed (Peat) and BASF granules (B. Granule) were supplied by BASF and New Edge Microbials supplied freeze dried inoculant (Freeze dried). Granules were supplied at the standard rate and two times the recommended rate (2 x RR Granule).
Despite a very dry finish at Wanilla which limited faba bean grain yields, all inoculants improved yields for the early sown treatments (Table 2). Novozyme granules increased yield at both sowing times, relative to peat slurry inoculation (Table 2). Notably the combination of peat slurry on seed and granules (BASF) also resulted in improved nodulation and yields when sown early. Late sown treatments produced less than half the yield of early sown treatments due to decreased growing degree days.
Table 2. Grain yields (t/ha) of Samira faba bean at Wanilla, SA in response to different inoculation treatments when sown into dry soil. Note that there was approximately four weeks between sowing and sufficient rainfall for germination after sowing 1 and approximately two weeks between sowing and sufficient rainfall for germination after sowing 2.
Inoculation treatment | Sowing Time | |
|---|---|---|
28 April (dry four weeks) | 16 June (dry two weeks) | |
Uninoculated | 0.13 ef | 0.07 g |
BASF peat | 0.30 d | 0.10 fg |
BASF peat and New Edge freeze dried | 0.30 d | 0.11 fg |
BASF granules | 0.39 c | 0.11 fg |
BASF peat + granules | 0.49 b | 0.14 ef |
Alosca granules | 0.33 cd | 0.11 fg |
Novozyme granules (tag team) | 0.57 a | 0.19 c |
l.s.d. | 0.06 | |
Inoculation with any formulation improved grain yield of lupin at either sowing time at Farrell Flat, SA (Table 3). In the dry sown treatments, peat slurry inoculation on seed provided the best inoculation response. However, crop yields were generally lower when sown after the break, most likely due to reduced degree days experienced by the crop. There were no differences among the different inoculant formulations for these treatments that were sown into moist soil (Table 3).
Table 3. Grain yields (t/ha) of narrow-leaf lupin at Farrell Flat, SA in response to different inoculation treatments sown either prior to the break or following breaking rains.
Inoculation treatment | Sowing Time | |
|---|---|---|
Dry sown (13 April) | Sown after break (28 April) | |
Uninoculated | 1.64 c | 1.86 c |
BASF peat | 2.90 a | 2.46 b |
BASF peat and New Edge freeze dried | 2.67 ab | 2.33 b |
BASF peat granules | 2.48 b | 2.35 b |
BASF peat + granules | 2.50 ab | 2.40 b |
BASF granules x 2 | 2.46 b | 2.35 b |
l.s.d. | 0.34 | |
Which additives reduce the efficacy of rhizobia?
Although Australian peat inoculants are considered to be the highest quality in the world (Hartley et al. 2005), nodulation potential can be quickly reduced through mixing with additives such as herbicides, fungicides, insecticides and/or fertilisers. The effects of these practices become obvious when a legume is sown on an inoculation responsive site. While it is recommended that mixing rhizobia with other amendments is best avoided, it is currently commonplace. The compatibility of rhizobial inoculants with some commonly used products is being tested in the laboratory to determine the effects of these additives on rhizobial survival. Some preliminary data indicated that zinc sulphate seed treatments are highly toxic to rhizobia (Ballard et al. 2016).
Recent laboratory tests also indicated that the presence of Gaucho® insecticide (imidacloprid) did not significantly reduce nodulation in field pea, relative to the water control (Figure 4). However, the application of either Thiram® (dimethyl dithiocarbamate) or P-Pickel T® (thiram and thiabendazole) both significantly reduced rhizobial numbers and nodulation. In chickpea, rhizobia numbers on seed and the resulting nodulation were both reduced by the addition of Gaucho insecticide, Thiram or P-Pickel T (Figure 4), indicating that chickpea rhizobia may be more sensitive than pea rhizobia to Gaucho.
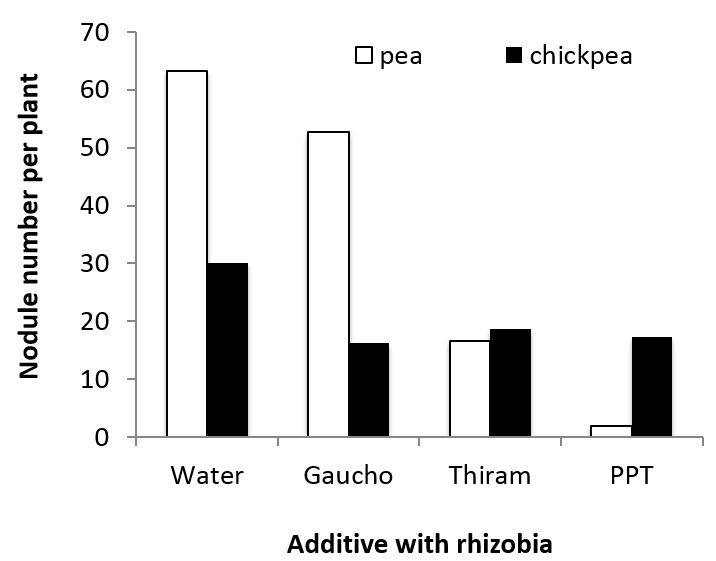
Figure 4. Number of nodules formed following the exposure of rhizobia on seed to different additives for 24 hours under laboratory conditions. Significant differences were observed among treatments for pea – l.s.d. 5% of 23; chickpea: l.s.d. 5% of 15.
Can new rhizobial strains improve nodulation in faba bean and lentil?
Rhizobia are typically much more sensitive to acid soils than the host legume plant. Improved acid tolerance in rhizobia that nodulate faba and lentil (along with pea and vetch) are likely to increase the area where these legumes can be grown. Promising strains of acid tolerant rhizobia for faba bean have been tested in 2017. The acid tolerant strains are showing encouraging results and the use of two strains, SRDI954 and SRDI969 have resulted in increased nodulation over the current commercial strain, WSM1455 (Figure 5). Strain SRDI954 has increased nodulation at five of the eight sites where it has so far been tested. Plans are also afoot to test the ability of these strains in improving the lentil symbiosis. It is envisaged that these new strains may enable expansion of pulses onto more acidic soils than has previously been possible.
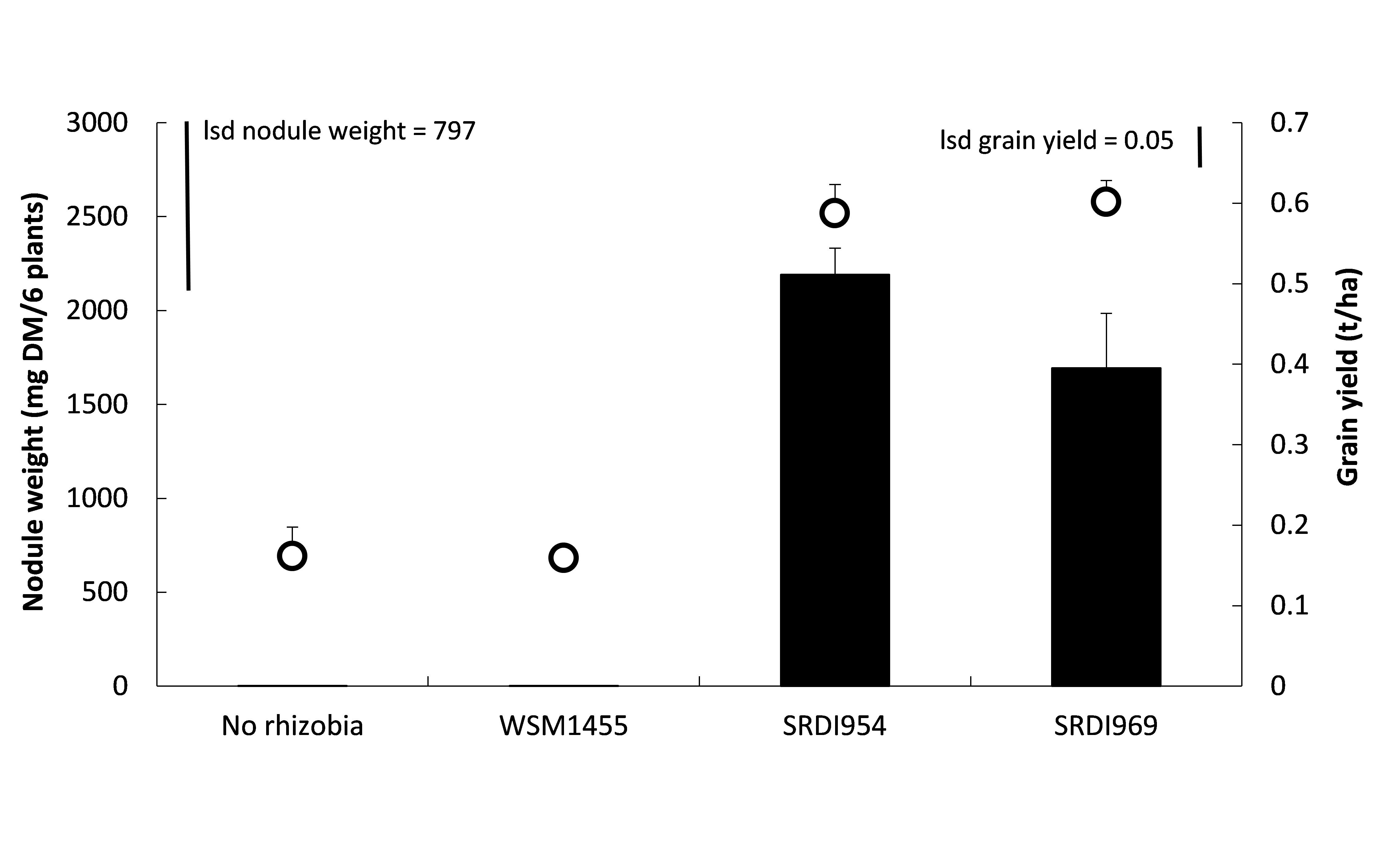
Figure 5. Effect of rhizobia strain on nodule weight (left axis, columns) and grain yield (right axis, circles) of Samira faba bean at Wanilla, Eyre Peninsula SA in 2017. Site pH(Ca) = 4.3, sown into dry soil at standard rates of inoculation on 28 April. Standard error of means shown as bars above columns and circles.
Conclusion
Peat slurry inoculant applications often provide the best nodulation when sown into moist soil, due to their high titres of rhizobia and the protective properties of peat, as well as proximity of the inoculant to the seed relative to other formulations and application methods. Granular inoculants and peat inoculants delivered using liquid injection have produced similar, sometimes even better results to peat applied to seed in some trials, but on the whole are less consistent in their performance. Since these alternative inoculation methods allow the inoculant to be separated from harmful seed-applied chemicals, it would be useful to better understand the conditions where they are effective.
Recognising that the dry sowing of pulses is a common practice despite its potential detriment on the symbiosis where there are no background rhizobia, efforts are underway to better understand the effects of dry sowing on rhizobial survival, so that improved recommendations can be provided to growers. Initial results indicate that some peat granules and traditional peat slurry inoculants applied to seed in high numbers produced satisfactory nodulation and grain yield in dry sowing conditions for faba bean and lupin. Further work is required to understand more broadly and conclusively how rhizobia survive and nodulate under dry sowing conditions. In the short term, results indicate that to optimise nodulation when dry sowing, rhizobia can be applied in high numbers at sowing by increasing the rate of inoculant applied (e.g. 2 x recommended rate of peat or granules, or applying both peat + granules together).
In practice, inoculation is often used in conjunction with other additives. Growers should be very wary when using additives such as fertilisers, seed-applied fungicides and organic products with rhizobia. The use of these products may lead to reduced rhizobial survival, nodulation, N fixation and grain yield.
New rhizobia for faba bean and lentil appear to provide greater acid tolerance than the current commercial strain of rhizobia. Commercialisation of these strains will lead to an improvement in soil adaptation of these crops.
Useful resources
Inoculating Legumes: A Practical Guide
References
Ballard R, Farquharson E, Ryder M, Rathjen J, Denton M (2016) Maximising the fixed nitrogen benefits of pulses. GRDC update, Adelaide.
Denton MD, Pearce DJ, Ballard RA, Hannah MC, Mutch LA, Norng S, Slattery JF (2009) A multi-site field evaluation of granular inoculants for legume nodulation. Soil Biology & Biochemistry 41, 2508-2516.
Denton MD, Pearce DJ, Peoples MB (2013) Nitrogen contributions from faba bean (Vicia faba L.) reliant on soil rhizobia or inoculation. Plant and Soil 365, 363-374.
Denton MD, Phillips LA, Peoples MB, Pearce DJ, Swan AD, Mele PM, Brockwell J (2017). Legume inoculant application methods: effects on nodulation patterns, nitrogen fixation, crop growth and yield in narrow-leaf lupin and faba bean. Plant and Soil, 419, 25–39.
Drew EA, Denton MD, Sadras VO, Ballard RA. (2012). Agronomic and environmental drivers of population size and symbiotic performance of Rhizobium leguminosarum bv. viciae in Mediterranean-type environments. Crop and Pasture Science. 63, 467-77.
Drew E, Herridge DF, Ballard RA, O’Hara GW, Deaker R, Denton MD, Yates RJ, Gemell G, Hartley E, Phillips L, Seymour N. (2014). Inoculating legumes: a practical guide. Grains Research and Development Corporation.
Hartley EJ, Gemell LG, Slattery JF, Howieson JG, Herridge DF (2005) Age of peat-based lupin and chickpea inoculants in relation to quality and efficacy. Australian Journal of Experimental Agriculture: 45, 183-188.
Unkovich MJ, Baldock J, Peoples MB (2010) Prospects and problems of simple linear models for estimating symbiotic N2fixation by crop and pasture legumes. Plant and Soil 329, 75-89.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. The support of SAGIT (project S217) is acknowledged for the work presented on dry sowing.
Contact details
Matthew Denton
Waite Campus, School of Agriculture, Food and Wine, University of Adelaide
PMB1 Glen Osmond SA 5064
08 8313 1098
matthew.denton@adelaide.edu.au
Researchers Adelaide profile
@_mattdenton
Was this page helpful?
YOUR FEEDBACK
