Blackleg in canola: an update on resistance, upper canopy infection and a new management App
Take home messages
- In 2017, blackleg leaf infection and resultant crown canker severity was low due to dry conditions in May and June during early seedling growth. Susceptible cultivars still had some level of disease but were well protected by fungicides applied at sowing which were highly effective due to the lower disease pressure. Application of foliar fungicides at the 4 to 6 leaf stage was generally not warranted.
- Upper Canopy Infection (UCI) is the collective term for blackleg flower, peduncle, pod, main stem and branch infection but does not include leaf lesions or crown canker.
- In 2016 and 2017, UCI caused large yield loss (up to 1t/ha) but the prevalent symptoms varied between years: pod lesions in 2016, stem and branch lesions in 2017.
- Delayed flowering after mid-August reduced severity of UCI in medium rainfall environments. Although flowering time is one important factor in the development of UCI, seasonal conditions relating to spore development and release, as well as infection events interact to produce UCI. Further research is required to understand and predict these interactions.
- Effective major gene resistance provides control of pod, branch and stem infection. Fungicides also control UCI however, further research is required to determine robust recommendations for foliar fungicide timing and determining the economic returns.
- The Blackleg Management App (‘BlacklegCM’ due for release February/March 2018) has been developed to provide growers and advisers with an interactive interface to explore the economic outcomes of different blackleg management strategies and their relative importance.
Blackleg resistance
There are two types of blackleg resistance genes in Australian canola cultivars; major and minor resistance genes. Major resistance genes stop the fungus from infecting the plant which results in complete protection against the blackleg pathogen. This is evidenced in the field by lack of leaf lesions and crown canker. A cultivar can have none, one or multiple major resistance genes. In Australia, all commercial canola cultivars are classified into Resistance Groups which describe the major genes present in the cultivar. For example, Group A cultivars have a single major resistance gene, Group ABDF cultivars have four major resistance genes. Blackleg is rapidly able to change and major gene resistance imposes strong selection for only those isolates of blackleg able to infect the plant. Therefore, major resistance genes can be overcome quickly in the field so cultivars dependent on major resistance genes tend to become more susceptible over time, sometimes becoming completely ineffective in as little as three years. Minor gene resistance (sometimes called quantitative, adult plant or crown canker resistance) reduces the severity of crown canker but does not inhibit leaf lesions and upper canopy infection.
As the name suggests, each gene provides a minor level of crown canker resistance. However, the combined effect of a number of minor genes in the same cultivar can create very high levels of crown canker resistance. As blackleg is able to infect plants with minor gene resistance, selection of isolates able to overcome these genes isn’t as strong, and therefore, can be robust for many years. At present, there is no rapid screening technique such as that used for major resistance genes, to identify the presence of minor resistance genes in cultivars. The Blackleg Rating classifies cultivars according to their overall level of resistance and includes both major and minor gene resistance. As major resistance genes inhibit infection of the plant, it is not until this resistance is overcome that it is then possible to determine the presence of minor resistance genes.
It is important to know the Resistance Group and also the Blackleg Rating when selecting a cultivar. In the field, cultivars with effective (not yet overcome) major gene resistance will be completely protected (no leaf lesions, crown canker or upper canopy infection). Crops with major gene resistance that has been overcome will be susceptible to leaf lesions and UCI but may still be resistant, or partially resistant to crown canker if minor resistance genes are present in the cultivar in combination with major resistance genes. An example of various levels of minor gene resistance is ATR Bonito and ATR Mako. Both cultivars are in Resistance Group A, and therefore, have the same major resistance gene which in many locations has been overcome (Table 1). The Blackleg Rating of ATR Bonito is moderately susceptible (MS) while ATR Mako has a higher rating of moderately resistant (MR). As these cultivars are not completely susceptible to blackleg, this indicates that minor and major resistance genes are present in combination but ATR Mako has better minor gene resistance.
In Australia, all cultivars classified in Resistance Group C have no effective major gene resistance and are therefore solely reliant on minor resistance genes with those with a higher Blackleg Rating more resistant to crown canker.
Periods of infection by blackleg for different plant parts
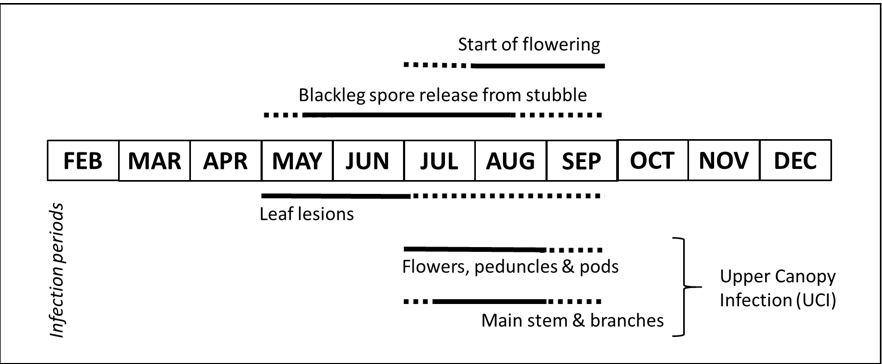
Blackleg is able to infect all parts of the canola plant. Figure 1 shows the relationship between the period of blackleg spore release and symptom development on different plant parts. Lesions form on leaves throughout the growing season however, severe crown canker is most likely to develop when plants are infected during the early seedling stage. The fungus grows from the cotyledons and leaves asymptomatically through the vascular tissues to the crown where it causes necrosis resulting in a crown canker at the base of the plant. Yield loss results from restricted water and nutrient uptake by the plant. Protection during the seedling stage is critical to reduce crown canker severity. Lesions can also develop on all other plant parts and these infections may go on to develop cankers as described further within this paper.
Winter is the main period in which conditions are generally most conducive for infection as rainfall triggers release of mature spores from crop residue and provides ideal conditions for the fungus to survive while it infects the crop (Figure 1). Once the plant has begun to flower, infection of flowers, peduncles, pods, main stem and branches of the plant has collectively been termed UCI (Figure 2). Any plant parts of susceptible cultivars exposed to spores during the winter period are likely to become infected and potentially cause yield loss. Upper canopy infection has become increasingly prevalent over recent years. Earlier flowering times and changes in farming systems with increased retention of stubble may contribute to higher disease severity. While the cost to yield and control of leaf lesions leading to crown canker is well understood, the factors contributing to UCI and possible control strategies are currently under investigation with current knowledge presented in this paper.
Figure 1. Periods of infection by blackleg for different parts of the canola plant in relation to the period of blackleg spore release and start of flowering in medium and high rainfall zones. Solid lines indicate main periods of infection and dashed lines indicate reduced risk from infection. For start of flowering, solid line indicates the optimal period in which yield is maximised while reducing disease risk.
Blackleg crown canker in 2017 — seedling leaf lesions and crown canker severity
Crown cankers result from infection of plants while they are in the early seedling stage usually during May and June. Dry conditions in this period in 2017 resulted in generally low levels of leaf infection resulting in reduced crown canker severity. Predominant use of canola cultivars from the same resistance group (e.g. Group A resistance) in the same locality or region results in blackleg populations with a high frequency of isolates virulent towards that group.
Since 2015, the Blackleg Rating of many of the Group A cultivars has fallen from MR to MS, indicating their increased susceptibility to disease. The severity of crown canker was assessed at 34 locations across the Australian canola-growing regions, and indicates the efficacy of different Resistance Groups (Table 1). Resistance Group H is not represented at these sites as winter cultivars are the only currently commercially available cultivars classified as Group H. At many locations, cultivars in multiple Resistance Groups had a high level of crown canker compared to the site mean. For example, Cootamundra in NSW had high levels of crown canker in Resistance Groups A, B, BF and AS. It should be noted that the cultivar used at these sites to represent Resistance Group C is ATR Stingray which has a good level of minor gene resistance with a Blackleg Rating of MR and hence low levels of crown canker (see preceding comments for further discussion).
Increased intensification of canola plantings in the past few years has resulted in large areas of canola stubble that can release blackleg spores with the potential to cause significant yield losses in years where spore release coincides with environmental conditions conducive to infection, such as those experienced in 2016. Despite the low disease pressure in 2017, leaf lesions were present in susceptible cultivars but were adequately controlled by fungicide treatments applied to seed or fertiliser at sowing, with foliar fungicide application generally unwarranted. In contrast, wet winter conditions in 2016 produced extreme levels of leaf lesions which warranted the application of foliar fungicides at the 4 to 6 leaf stage to extend the efficacy of seed and fertiliser treatments.
Table 1. Blackleg crown canker severity in cultivars from different Blackleg Resistance Groups at monitoring sites in 2017. Disease severity is indicated as high, moderate or low compared to the site average.
Monitoring | Resistance Group | |||||||
|---|---|---|---|---|---|---|---|---|
Group A | Group B | Group C | Group AD | Group ABD | Group ABDF | Group BF | Group AS | |
NSW | ||||||||
Beckom | High | High | Low | Low | Low | Low | High | High |
Bellata | Low | Low | Low | Low | Low | Low | Low | Low |
Cootamundra | High | High | Low | Low | Low | Low | High | High |
Cudal | High | High | Low | Low | Low | Low | Low | Low |
Gerogery | Mod | High | Low | Low | Low | Low | High | High |
Grenfell | High | High | Low | Low | Low | Low | Low | Low |
Lockhart | High | High | Mod | Low | Low | Low | High | Mod |
Mullaley | Low | High | Low | Low | Low | Low | High | Low |
Parkes | High | High | Low | Low | Low | Low | Low | Low |
Tamworth | High | High | Low | Low | Low | - | Mod | Low |
Wagga Wagga | High | High | Low | Low | Low | Low | Mod | Low |
SA | ||||||||
Arthurton | High | Low | Low | Low | Low | Mod | High | High |
Bordertown | High | Low | Mod | High | Mod | Mod | High | High |
Cummins | Low | Low | Low | High | Low | Low | Low | Low |
Frances | High | Low | Low | Mod | Low | Mod | High | High |
Mt Hope | High | Low | Mod | Mod | Low | Mod | High | Mod |
Riverton | High | Low | Low | High | Low | Low | Low | Low |
Spalding | High | Low | Low | High | Mod | Low | Low | High |
Turretfield | Mod | Low | Low | Mod | Low | Low | High | High |
Wangary | High | Low | Low | Mod | Low | Low | High | Low |
Yeelanna | High | Mod | Mod | Low | Low | Mod | High | Mod |
VIC | ||||||||
Charlton | Low | High | Low | Low | Low | Low | High | Low |
Diggora | High | High | Low | Mod | Mod | Low | High | High |
Cavendish | Low | Low | Low | Low | Low | Low | Low | Mod |
Kaniva | Mod | High | Low | Low | Low | Low | High | Mod |
Minyip | High | Mod | Low | Low | Low | Low | Mod | High |
Streatham | Mod | High | Low | High | Low | Low | Low | High |
Yarrawonga | High | High | Mod | Low | Low | Low | High | Mod |
WA | ||||||||
Corrigin | Mod | High | Low | Low | Low | Low | High | Mod |
Gibson | High | Mod | Mod | Low | Low | Low | Mod | Mod |
Katanning | Mod | High | Low | Low | Low | Low | High | Low |
Kendenup | High | Low | Low | Low | High | High | Low | High |
Kojonup | Low | High | Low | Low | Low | Low | High | Low |
Williams | High | Mod | Low | Low | Low | Low | Mod | Mod |
Upper canopy infection
The infection by blackleg of flowers, peduncles, pods, stems and branches is termed UCI. In 2010, cankers on the upper stems and branches were observed in commercial canola paddocks (Figure 2). These cankers appeared to cause yield loss as the pods on affected branches senesced prematurely leading to early pod shatter. Stem/branch cankers are not correlated with the presence of crown cankers. In 2011, 2012 and 2013 stem/branch cankers were observed each year but symptoms were not generally severe and were not present in all regions. In 2014 and 2015, the symptoms were widespread and appeared to cause substantial yield loss. In 2016, research commenced investigating the causes and management of UCI. In contrast to previous years, severe stem/branch infection was not present at most sites in 2016. Data from 2016 clearly showed that flowering during the winter period where conditions for blackleg infection are optimal, consistently resulted in increased UCI. The data also clearly showed that UCI caused large yield losses (data not presented within paper).

Figure 2. Upper canopy infection includes blackleg infection of flowers, peduncles, pods, main stems and branches.
UCI yield loss
Field experiments conducted in 2016 and 2017 show that in the absence of sclerotinia or blackleg crown canker, UCI caused yield loss of up to 1t/ha in southern NSW compared to where disease was fully controlled (Figure 3). In both seasons, delaying the onset of flowering after mid-August reduced yield loss. However, in 2016 crops starting to flower in early August had minimal yield loss compared to those in 2017 that had 0.7t/ha yield loss. This data indicates that although flowering time is one factor important in the development of UCI, seasonal conditions will determine the prevalence and severity of UCI. Spore development and release, as well as infection events interact with crop development stage to produce varying severity of UCI. Further research is required to understand and predict these interactions.
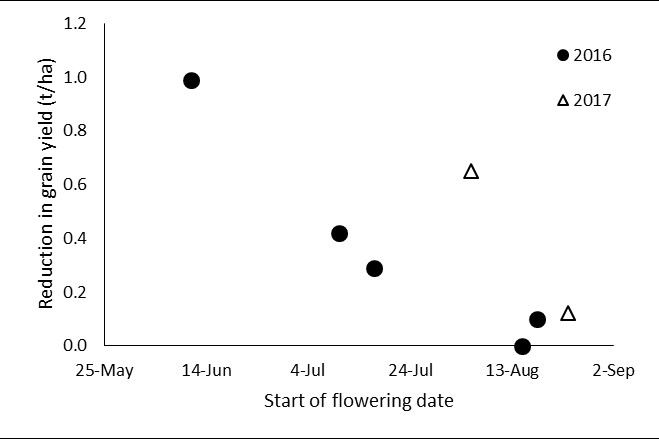
Figure 3. Yield loss caused by Blackleg UCI in cultivar Pioneer®44Y89CL differing in start of flowering date in southern NSW in 2016 and 2017. Yield reduction was the difference between treatments with none or full disease control.
Infection of pods by blackleg can cause complete loss of pods as they break off the plant or shatter prematurely. Grain inside infected pods retained on the plant can also be affected (Table 2). Pods with increasing severity of blackleg lesions have reduced grain size and may have fewer seeds/pod. Severe blackleg lesions (>10mm) reduced grain size by up to 22% indicating that the effects of pod infection occur after seed number is set but that seeds may be aborted if directly infected. In addition, fully formed seed within infected pods and which is retained for future use is infected with blackleg. Plants growing from infected seed can have seedling blight resulting in poor crop establishment. Given the high level of pod infection by blackleg and alternaria (both of which can cause seedling blight) it is recommended that seed from crops with infected pods is not retained for sowing. If retaining seed, grade it for larger seed which is less likely to be infected with blackleg and ensure even and adequate treatment with an appropriate fungicide to control seedling blight.
Table 2. The yield components of individual pods with blackleg pod lesions at Canowindra and Wagga Wagga (cv Pioneer®44Y89CL), NSW in 2016 and Horsham (ATR-Stingray), VIC in 2017. TGW = thousand grain weight. Values followed by the same letter within each column are not significantly different (P<0.05).
Pod blackleg lesion size | New South Wales 2016 | Victoria 2017 | ||||
|---|---|---|---|---|---|---|
Canowindra, NSW | Wagga Wagga, NSW | Horsham, VIC | ||||
TGW (g) | Seeds/pod | TGW (g) | Seeds/pod | TGW (g) | Seeds/pod | |
No lesions | 3.69a | 19.3a | 3.43a | 23.4a | 2.98a | 22.6a |
<3mm | 3.57b | 19.8a | 3.26ab | 21.7b | 3.23a | 20.5a |
3-5mm | 3.45c | 19.0a | 3.26ab | 21.4b | 3.14a | 19.9a |
5-10mm | 3.37cd | 19.3a | 3.20bc | 20.4c | 2.89a | 21.3a |
>10mm | 3.17d | 18.5a | 3.06d | 18.8d | 2.33b | 20.8a |
In contrast to pod lesions which directly affect the developing seed, branch lesions cause a disruption to the flow of nutrients to the developing pods and seeds. In 2017, branch lesions reduced the number of seeds/pod and also seed weight (Table 3). Consistent effects were found in three cultivars with different flowering times: Nuseed Diamond, Pioneer®44Y89CL and Archer.
Table 3. The yield components of pods harvested from the main raceme of plants with blackleg branch lesions at Wagga Wagga (cvs. Pioneer®44Y89CL, Nuseed Diamond and Archer) in 2017. Pods were collected above the lesions on each plant. The data are the mean of the cultivars as their response to disease was the same. TGW = thousand grain weight. Values followed by the same letter within each column are not significantly different (P<0.05).
Severity of blackleg branch lesion | TGW (g) | Seeds/pod |
|---|---|---|
No lesion | 3.73ab | 12.63a |
Moderate | 3.92a | 11.35b |
Severe | 3.59bc | 9.70c |
Control of UCI — what we know and don’t know
In 2017, experiments were conducted to confirm the effects of flowering time, cultivar resistance and fungicide use/timing. The results can be summarised as the following:
- Pod infection was the predominant symptom present in experiments in 2017. Later flowering reduced disease severity (Table 4).
- In 2016 levels of UCI infections were low in plants that flowered after July. However, in 2017 although symptoms were reduced in plants that flowered after July there was still some severe pod infection and UCI in late flowering plants (Table 4 and Table 6).
- Cultivars with effective major gene resistance do not get UCI symptoms, including pod infection (e.g. Group ABDF) (Table 4). Cultivars in Resistance Group C have no effective resistance to UCI in Australia. In these cultivars, the Blackleg Rating indicates resistance to crown canker only, not UCI. Although ATR-Stingray has higher levels of UCI than Archer, this is solely due to the earlier flowering time of ATR-Stingray.
- Fungicides applied during the reproductive growth stages will reduce the severity of UCI. However, the economics of fungicide application at this stage are yet to be determined. The economic return will depend on the severity of symptoms and the timing of fungicide application. For example, ATR-Stingray sown in March will have a greater return from fungicide application compared to ATR-Stingray sown in June as it would flower outside the critical window for infection. Archer (later flowering cultivar) did not get enough UCI to warrant control regardless of sowing time. Hyola®350TT (Group ABDF) with effective major gene resistance did not get enough UCI to warrant control regardless of sowing time (Table 5 and Table 6).
- Data indicates that spraying at 30% bloom for sclerotinia control may reduce UCI symptoms if the season is conducive to disease development (Table 5 and Table 6).
- There is insufficient knowledge to recommend spraying solely for UCI control.
- In both 2016 and 2017 the yield responses to controlling UCI appear to be greater than the reduction in levels of visible symptoms of UCI. That is, small reductions in symptoms have resulted in significant yield increases. Infection by the blackleg fungus does not always produce visible symptoms. Symptomless infection of the crown can cause significant damage to the plant vascular tissue, and evidence suggests that branch and stem infection is similar. Further research is required to understand how UCI is causing yield losses (Table 5 and Table 6).
Table 4. 2017 Effect of flowering date and cultivar resistance of upper canopy infection symptoms. Experiment undertaken in pots with canola stubble spread around the pots to ensure disease inoculation at Horsham, VIC.
Cultivar + date 1st flower | % flower infection | Stem | Branch | % head | % pod infection | % crown canker |
|---|---|---|---|---|---|---|
| ATR-Stingray | MR + Group C ineffective major gene in this experiment | |||||
9-Jul | 2.8 | 0.2 | 0.5 | 0.3 | 33 | 0 |
30-Jul | 1.5 | 0.1 | 0.7 | 0.2 | 27 | 1 |
25-Aug | 1.5 | 0.1 | 0.7 | 0.1 | 3 | 8 |
10-Sep | 0.8 | 0.1 | 0.2 | 0.0 | 1 | 12 |
Archer | MS + Group C (ineffective major gene in this experiment) | |||||
14-Aug | 0.2 | 0.6 | 0.6 | 0.2 | 4 | 5 |
29-Aug | 0.0 | 0.5 | 0.6 | 0.1 | 3 | 22 |
9-Sep | 1.0 | 0.2 | 0.6 | 0.1 | 1 | 37 |
19-Sep | 0.5 | 0.1 | 0.1 | 0.0 | 0 | 38 |
Nuseed Diamond | R + Group ABF (partially effective major gene in this experiment) | |||||
28-Jun | 2.8 | 0.1 | 0.4 | 0.2 | 7 | 1 |
20-Jul | 0.3 | 0.0 | 0.2 | 0.1 | 17 | 1 |
13-Aug | 1.5 | 0.0 | 0.5 | 0.1 | 2 | 12 |
8-Sep | 0.5 | 0.0 | 0.5 | 0.0 | 1 | 7 |
Nuseed GT42 | Group ABDF (immune in this experiment) | |||||
7-Jul | 0.0 | 0.0 | 0.0 | 0.0 | 2 | 0 |
7-Aug | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 0 |
2-Sep | 0.0 | 0.1 | 0.0 | 0.0 | 0 | 1 |
16-Sep | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 1 |
Hyola®350TT | Group ABDF (immune in this experiment) | |||||
7-Jul | 0.0 | 0.0 | 0.0 | 0.0 | 2 | 0 |
27-Jul | 0.2 | 0.0 | 0.0 | 0.0 | 0 | 0 |
25-Aug | 0.7 | 0.0 | 0.0 | 0.0 | 0 | 2 |
9-Sep | 0.3 | 0.0 | 0.0 | 0.0 | 0 | 1 |
The 0 to 4 scale: 0=no symptoms, 1=symptoms present, 2=symptoms common, 3=symptoms causing significant damage, 4=stem or branch death.
Table 5. 2017 Effect of flowering date and fungicide application on upper canopy infection symptoms and yield. Experiment undertaken in pots with canola stubble spread around the pots to ensure disease inoculation. UT = untreated, Full = full control. Cultivar ATR-Stingray.
Fungicide | % flower infection | Stem | Branch | Head | % pod infection | % crown canker | Yield % of untreated |
|---|---|---|---|---|---|---|---|
1st flower 9 July | |||||||
Untreated | 1.7 | 0.6 | 0.2 | 0.58 | 34 | 0 | 100 |
30% bloom | 0.8 | 0.2 | 0.0 | 0.30 | 34 | 0 | 116 |
Full | 0.2 | 0.1 | 0.0 | 0.01 | 2 | 0 | 155 |
1st flower 25 August | |||||||
Untreated | 3.7 | 0.9 | 0.2 | 0.10 | 4 | 4 | 100 |
30% bloom | 1.7 | 0.3 | 0.0 | 0.02 | 2 | 5 | 97 |
Full | 1.3 | 0.1 | 0.0 | 0.02 | 1 | 5 | 116 |
The 0 to 4 scale: 0=no symptoms, 1=symptoms present, 2=symptoms common, 3=symptoms causing significant damage, 4=stem or branch death.
Table 6. 2017 Effect of flowering date and fungicide application on upper canopy infection symptoms and yield. Experiment undertaken in the field at Longerenong, VIC, sown in plots exposed to natural blackleg inoculum. There were only very low levels of blackleg crown canker, no sclerotinia and no other diseases present.
Fungicide | Cultivar | ||
|---|---|---|---|
ATR-Stingray | ATR-Gem | ATR-Wahoo | |
1st flower date | |||
Sown 14-April | 17 Aug | 13 Aug | 28 Aug |
% flowers infected | |||
Untreated | 12 | 10 | 11 |
30% bloom | 5 | 7 | 9 |
Full control | 5 | 0 | 0 |
Stem infection (0-4 scale, 4 =stem death) | |||
Untreated | 0.5 | 0.9 | 0.4 |
30% bloom | 0.1 | 0.6 | 0.4 |
Full control | 0.2 | 0.1 | 0.2 |
Branch infection (0-4 scale, 4 =branch death) | |||
Untreated | 1.2 | 1.1 | 0.9 |
30% bloom | 0.5 | 0.5 | 1.0 |
Full control | 0.5 | 0.2 | 0.2 |
% pods infected | |||
Untreated | 13 | 20 | 14 |
30% bloom | 10 | 6 | 6 |
Full control | 6 | 3 | 5 |
Yield (% of untreated control) | |||
Untreated | 100 | 100 | 100 |
30% bloom | 110 | 112 | 108 |
Full control | 118 | 112 | 121 |
The 0 to 4 scale: 0=no symptoms, 1=symptoms present, 2=symptoms common, 3=symptoms causing significant damage, 4=stem or branch death.
Blackleg management App — BlacklegCM
The current Blackleg Management Guide contains information on the management factors that influence the severity of blackleg in your crop. It specifically lists cultural practices such as crop rotation and distances to canola stubble (inoculum source), the appropriate scenarios for fungicide application and presents the Blackleg Rating and Resistance Group for each cultivar. The Management Guide is updated twice yearly as the resistance status of individual cultivars can change as the blackleg fungus overcomes host resistance genes.
Although the Management Guide provides useful information, it has some limitations in its current form. Currently, it is difficult to consider complex interactions. For example, the use of cultivars with different Blackleg Ratings in high or low rainfall environments and the effect of fungicide use. Consequently, there has been a need to develop a management tool that can provide disease forecasting based on the management principles proposed by the manager of an individual paddock. This has led to the development of the new Blackleg Management App. ‘BlacklegCM’.
BlacklegCM assists you to manage blackleg disease in Australian canola crops by integrating the information provided in the Blackleg Management Guide and producing a predicted economic outcome. BlacklegCM can be modified to account for some of the major factors that relate to risk of yield loss due to blackleg disease in your paddock. It allows you to compare the likely profitability of different disease management strategies including paddock selection, cultivar choice, seed dressing, banded fungicide and sprayed fungicide.
BlacklegCM takes account of costs, yield benefits and grain prices to give you best case, worst case and most likely estimates of economic return.
BlacklegCM accounts for the major factors that influence blackleg severity. The user has the option to change each parameter to tailor the output to their cropping circumstance. Consequently, the user can explore their options for disease control and understand the relative importance of each factor. For example, distance to one year old stubble has a large influence on disease severity, while two year old stubble has a minor influence. Foliar fungicide has a small influence if used in isolation but is very effective if used in combination with a seed dressing fungicide. Foliar fungicide on a one tonne crop is likely to cause an economic loss while fungicide on a three tonne crop is more likely to result in a large profit.
The strength of the App is that it allows the user to make as many comparisons as they wish in order to determine the best and most profitable way for them to reduce disease and increase profits.
The App is a result of 30 years of blackleg research. It has had input from all members of the GRDC investment ‘National canola pathology program’ and has been built by the ‘National pathogen management modelling and decision support project’. The App has already been extensively tested by advisers and the interfaces were determined based on advisers’ recommendations (Figures 4, 5 , 6 and 7) .
BlacklegCM App loads with many options. The user can set these options to best match their circumstance (Figure 4).
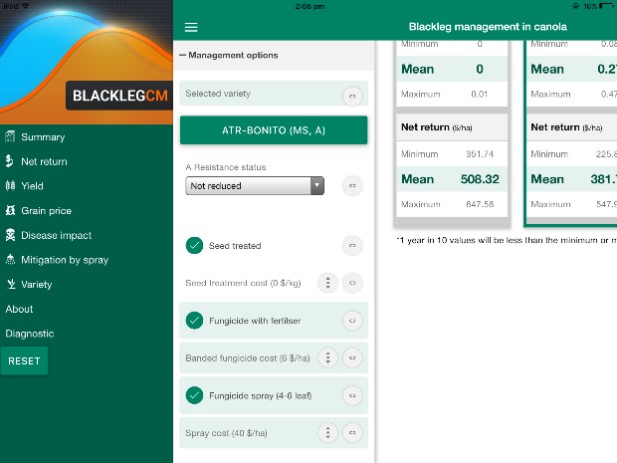
Figure 4. Options available with use of BlacklegCM App.
Crop circumstances
The user puts in basic parameters such as target yield, production costs, grain price, and regional canola intensity (Figure 5).

Figure 5. BlacklegCM App interface to collect information regarding crop circumstances.
Paddock set up
Within the paddock set up section, it’s possible for the user to fill in distance to one and two year old stubble and whether the stubble has been left standing or has been knocked down (Figure 6).
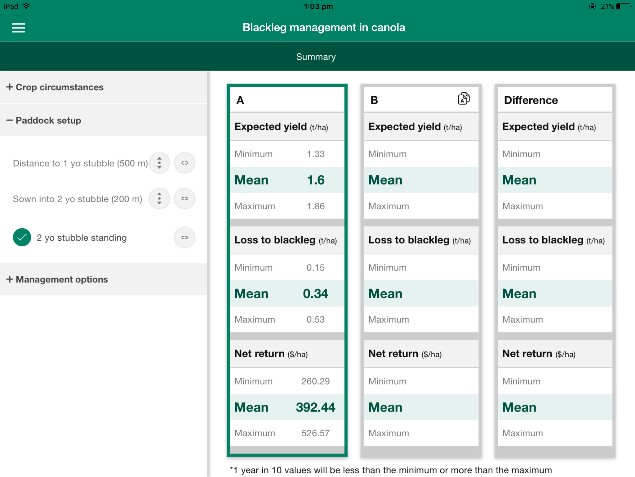
Figure 6. BlacklegCM App interface to collect information regarding paddock set up.
Management options
Within the management options section the user is able to choose your cultivar, and indicate whether your cultivar has reduced resistance in your region. This can be determined from monitoring past crops, but if unknown the App will default to ‘Not reduced’. If major resistance changes have occurred there will be published warnings, such as ‘Group D resistance warning on the Eyre Peninsula in 2012’.The management options section also enables the user to add their fungicide plans, seed treatment, fertiliser amended or foliar application (Figure 7).

Figure 7. BlacklegCM App interface to collect information regarding management options.
Once all of the parameters have been entered, the real power of the App becomes apparent as it determines the likely blackleg severity, yield loss and economic return from the parameters that have been entered. But unlike the current paper management guide, the App can calculate an immense number of interactions. For instance, in a low rainfall environment the App will determine that most management options do not results in yield loss and fungicide use may even result in economic loss. Whereas in the high rainfall, high canola intensity regions even small changes in management may result in varying levels of disease. The App also enables the user to compare different management options.
Case study
In 2018 a grower has ATR-Bonito seed which formerly had a blackleg rating of MR, however, it has fallen to Blackleg Rating MS. Should the grower use their ATR Bonito seed as intended or get new seed of a more resistant cultivar?
The grower puts in their parameters:
- Potential yield: 2t/ha
- Seeding rate: 3kg/ha
- Grain price: $500/t
- Production cost: $400/ha
- Canola in the district: 20%
- Spore maturity risk: High
- Distance to one year old stubble: 10metres
- Distance to two year old stubble: 200meters
- Two year old stubble: standing
- Cultivar: ATR Bonito
- Seed treatment: No
- Fungicide with fertiliser: No
- Fungicide spray: No
- The predicted yield loss from blackleg is 20%.
- The grower can now change parameters:
- New cultivar with R rating = 20% yield loss reduced to 0% yield loss.
- ATR Bonito with seed dressing and foliar fungicide = 20% yield loss reduced to 4% yield loss.
- ATR Bonito sown with increased distance to one year old stubble = 20% yield loss reduced to 10% yield loss.
The App will also be updated continuously to ensure that it has all the current canola cultivars and their current blackleg rating. All new knowledge will also be incorporated; for instance, knowledge on UCI and different fungicide timings will be incorporated in the near future.
The App can also be used during the growing season, for instance in 2016 many growers planned for a 2t/ha crop but soon realised that yield potentials were much higher. Members of the canola pathology team then warned of a very high blackleg lesion severity. In this scenario in July, growers could have re-run the App with 3t/ha rather than 2t/ha yield target and compared plus or minus foliar fungicide.
It is envisaged that this App will continue to grow and evolve with the canola industry and become the mainstay for blackleg knowledge in Australia.
Useful resources
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial co-operation and support of the GRDC. The authors would like to thank them for their continued support.
Contact details
Steve Marcroft
Marcroft Grains Pathology, Grains Innovation Park, Natimuk Rd, Horsham, VIC 3400
(03) 5381 2294, 0409 978 941
Steve@grainspathology.com.au
Susie Sprague
CSIRO Agriculture and Food, Canberra, ACT 2600
(02) 6246 5387, 0466 643 227
Susan.Sprague@csiro.au
Was this page helpful?
YOUR FEEDBACK