Herbicide and weed management - latest research
Author: Christopher Preston, David Brunton, Alicia Merriam, Hue Thi Dang, The Duc Ngo, Peter Boutsalis, Mahima Krishnan, Jenna Malone and Gurjeet Gill.(School of Agriculture, Food & Wine, University of Adelaide). | Date: 20 Feb 2018
Take home messages
- Annual ryegrass with resistance to the Group D and Group J pre-emergent herbicides will make management in cereals difficult.
- Indian hedge mustard populations have resistance to Groups B, C, F and I herbicides, greatly reducing control options in break crops.
- Common sowthistle with resistance to imidazolinone (IMI) herbicides is difficult to control in lentil crops, so control has to occur in cereals.
- Windmill grass and feathertop Rhodes grass are two emerging summer weeds with glyphosate resistance. Better understanding of their emergence and growth patterns will aid control.
Resistance to pre-emergent herbicides in annual ryegrass
With resistance to post-emergent herbicides in grass weeds, particularly in annual ryegrass, there has been increased reliance on pre-emergent herbicides for weed control. Pre-emergent herbicides are now one of the most important components of annual ryegrass management. Resistance to trifluralin has been present in South Australia (SA) for many years and by 2005, resistance to trifluralin was widespread. This resulted in early adoption of Boxer Gold® when it was released in 2008 and of Sakura® in 2012. The heavy dependence on Group J herbicides in recent years has led to resistance to this mode of action. Resistance to Group J herbicides in annual ryegrass has occurred in SA, VIC and NSW. In all cases, the populations also have resistance to trifluralin, suggesting that once trifluralin has failed, selection pressure shifts to other pre-emergent herbicides. Due to the existing widespread resistance to trifluralin in annual ryegrass in SA, resistance to the Group J herbicides will leave one mode of action available for control of annual ryegrass in cereals.
The situation may be even worse than this. In one population of annual ryegrass from the Eyre Peninsula with resistance to Group J herbicides that has been well characterised, resistance occurs across many herbicides of this mode of action (Table 1). As expected, this population also has resistance to trifluralin. More concerning, there is a reduction in susceptibility to both propyzamide and pyroxasulfone (Sakura®). The current ability to manage annual ryegrass with pre-emergent herbicides could be threatened if more populations like this appear.
Table 1. Concentration of various pre-emergent herbicides required for 50% mortality (LD50) of example resistant and susceptible annual ryegrass populations with resistance index (RI).
Annual ryegrass population | ||||
|---|---|---|---|---|
Herbicide (with Group) | SLR4 (S) | VLR1 (S) | EP162 (R) | RI* |
LD50 (g a.i. ha-1) | ||||
Triallate (J) | 248 | 181 | 3188 | 14.9 |
Prosulfocarb (J) | 311 | 246 | 2608 | 9.4 |
EPTC (J) | 305 | 288 | 2867 | 9.7 |
Trifluralin (D) | 39 | 27 | 455 | 13.8 |
Propyzamide (D) | 30 | 23 | 74 | 2.7 |
Pyroxasulfone (K) | 9.5 | 6.2 | 64 | 8.1 |
*RI = LD50 of R population divided by LD50 of S populations.
Multiple resistance in Indian hedge mustard
Indian hedge mustard has been a problematic broadleaf weed in SA for some years. It evolved resistance to the Group B herbicides early and in recent years, populations with resistance to 2,4-D, atrazine and diflufenican have been identified. Resistance to all of these herbicides is turning up in the random weed surveys being conducted across SA and VIC (Table 2). The frequency of samples with resistance to the Group C and Group F herbicides appears to be increasing rapidly.
Table 2. Extent of herbicide resistance in Indian hedge mustard populations from SA and VIC. Samples collected randomly at harvest from single fields. Resistant samples had greater than 20% survival at the normal field rate in testing.
Herbicide (with Group) | Samples with resistance (%) | ||
|---|---|---|---|
| 2013 Mid-North, SA | 2014 Eyre Peninsula, SA | 2015 Wimmera/Mallee, VIC | |
| Chlorsulfuron (B) | 25 | 64 | 37 |
| Imazamox + Imazapyr (B) | 13 | 14 | 5 |
| Atrazine (C) | 0 | 7 | 32 |
| Diflufenican (F) | 0 | 36 | 37 |
| 2,4-D (I) | 0 | 7 | 16 |
| Glyphosate (M) | 0 | 0 | 0 |
Multiple resistance across all of Groups B, C, F and I was also present (Table 3). Of the 50 populations collected in random surveys since 2013, only 38% were susceptible to all herbicides, 36% had resistance to one mode of action, 18% had resistance to two modes of action, 6% had resistance to three different modes of action and one population had resistance to all four modes of action.
Table 3. Extent of multiple herbicide resistance in 50 Indian hedge mustard populations from SA and VIC collected randomly at harvest between 2013 and 2015.
Herbicide Groups with resistance | Samples (% of total) |
|---|---|
Susceptible to all | 38 |
B | 22 |
C | 2 |
F | 10 |
I | 2 |
B + C | 2 |
B + F | 10 |
C + F | 6 |
B + C + F | 4 |
B + F + I | 2 |
B + C + F + I | 2 |
Clearly, this multiple resistance will make managing Indian hedge mustard more difficult. There remain some herbicide options that are still effective, in particular herbicide mixtures with bromoxynil seem to be providing effective control in cereals, however, options for pulse crops are limited. Crop topping can be used to reduce seed set in canola and pulse crops. Good control will have to be achieved in the cereal phase to reduce the weed seed bank heading into break crops.
Herbicide resistance in common sowthistle
Common sowthistle is another broadleaf weed species that has been increasing in importance recently due to herbicide resistance. Common sowthistle, Indian hedge mustard and prickly lettuce, evolved resistance to the Group B herbicides in SA early. Initially, Group B resistance in common sowthisle was restricted to the sulfonylurea (SU) herbicides. More recently, populations with resistance to IMI herbicides have appeared. Our most recent random resistance survey found nearly as much resistance to IMI herbicides (63% of populations) as to SU herbicides (72% of populations). The selection of IMI resistance has been the result of increased use of Clearfield® crops and associated IMI herbicides, particularly with lentils in SA.
Common sowthistle has also evolved resistance to Group I herbicides in SA. Resistance has occurred across the Group I herbicides, such as 2,4-D and clopyralid (Lontrel®). Resistance to glyphosate in this species has also appeared in NSW and QLD. Resistance to Group I herbicides in common sowthistle reduces the number of options for control both in crop and during summer fallow. With resistance to Group B, Group I and glyphosate, all of the inexpensive summer control options are gone.
The increasing prevalence of herbicide resistance in common sowthistle is a major reason why it is becoming difficult to manage in lentil production. The good news is that sowthistle has a short-lived weed seed bank of little more than 12 months. This means that effective control in cereal crops should be able to reduce problems in subsequent lentil crops. As there are few remaining effective control methods for sowthistle in lentils, growing lentil crops close together in the rotation will exacerbate sowthistle problems.
While common sowthistle seed is blown by wind and a small amount can move a long way, most of the seed falls within 100m of the parent plant. Spring germinating common sowthistle struggles to establish in competitive cereal crops and growing more competitive cereals will reduce the amount of sowthistle plants that can persist through harvest. Ensuring surviving plants do not set seed in crop fields, as well as pastures and fence lines, will have a large impact in reducing populations for the next cropping season. Common sowthistle is still susceptible to some herbicides and herbicide mixtures from Groups C, G, H and L. These are typically more expensive options, but using them in some paddocks to take the pressure off other modes of action will help.
Glyphosate resistance in feathertop Rhodes grass and windmill grass
Feathertop Rhodes grass and windmill grass are two summer-growing grass species that are starting to be more problematic in grain cropping in southern Australia. These are spring germinating species that have natural tolerance to glyphosate, although glyphosate can control both species if applied to small seedlings at robust rates. Both of these species are present in SA, but mainly on roadsides. Despite having tolerance to glyphosate, both species have also evolved resistance to glyphosate. Roadside populations of both species with glyphosate resistance have been identified and these move easily into cropped fields.
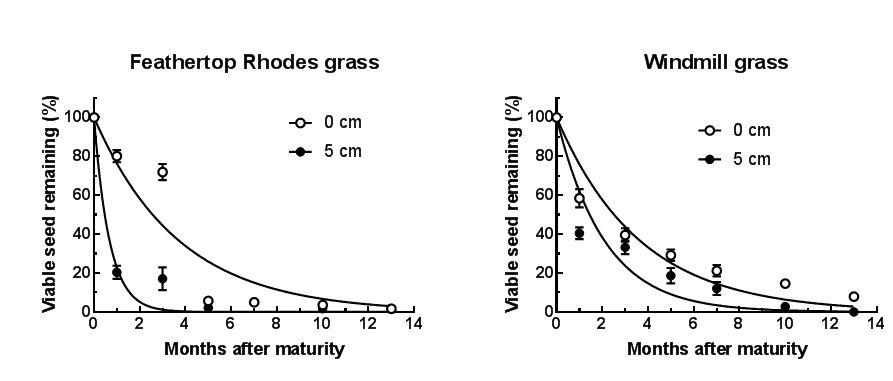
Feathertop Rhodes grass is an annual grass species that germinates mainly in spring and early summer in SA. Seasons with higher spring rainfall, such as 2016, will have larger flushes of germination. Feathertop Rhodes grass grows rapidly after germination and can set seed within two months in summer. Each plant can produce up to 40,000 seeds and seeds can move up to 30m by wind. Transport of seeds is aided by vehicles and farm equipment. The soil seed bank persists for about 12 months (Figure 1), but lasts longer following wetter summers. Persistence is greater for seed on the surface than for buried seed.
The ideal time to control feathertop Rhodes grass is when seedlings are small. Unfortunately, the common pre-emergent herbicides used in SA grain production are not effective at reducing feathertop Rhodes grass germination in spring. This means that control needs to occur as soon after harvest as possible and before weeds set seed.
Windmill grass is a slower growing plant than feathertop Rhodes grass taking longer to reach maturity. However, windmill grass is a short-lived perennial species and while seedlings can be controlled relatively easily, mature plants are very difficult to kill with herbicides. Like feathertop Rhodes grass, windmill grass typically germinates in spring in SA. The soil seed bank life of windmill grass is longer than feathertop Rhodes grass (Figure 1).
Windmill grass seed is less mobile than feathertop Rhodes grass and so new infestations are likely to be seen close to roadside fences. Controlling these infestations early before they fully establish is essential to reduce the costs of later control.
Figure 1. Persistence of seed of feathertop Rhodes grass (left) and windmill grass (right) on the soil surface (○) or buried at 5cm (●). From Ngo et al. (2017a; 2017b).
Useful resources
Common weeds of cropping - common sowthistle
Feathertop Rhodes grass biology
References
Ngo, T.D., Boutsalis, P., Preston, C. and Gill, G., 2017a. Plant Development and Seed Biology of Windmill grass (Chloris truncata) in Southern Australia. Weed Science, 65(3), pp.395-405.
Ngo, T.D., Boutsalis, P., Preston, C. and Gill, G., 2017b. Growth, Development, and Seed Biology of Feather Fingergrass (Chloris virgata) in Southern Australia. Weed Science, 65(3), pp.413-425.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the author would like to thank them for their continued support.
Contact details
Christopher Preston
University of Adelaide
(08) 8313 7237
christopher.preston@adelaide.edu.au
Was this page helpful?
YOUR FEEDBACK