Improving grain yields on hostile soils by combining genetic solutions and management of subsoil constraints
Author: Ehsan Tavakkoli, Zhe (Han) Weng, Binbin Xu (New South Wales Department of Primary Industries, Wagga Wagga Agricultural Institute, Wagga Wagga) Glenn McDonald (School of Agriculture, Food and Wine, Waite campus, The University of Adelaide, Glen Osmond) | Date: 13 Feb 2018
Take home messages
- The GRDC project aims to identify wheat cultivars with high grain yields in sodic soils and to determine the plant traits contributing to this yield.
- Significant genotypic variation in yield of wheat varieties was found when grown under multiple soil constraints over three seasons.
- Parallel screening of multiple stresses, rapid field-based phenotyping, the development of a rapid root screen and the ability to screen in soil-based systems under controlled conditions using ‘model’ sodic soils representative of the target environments will generate valuable knowledge for wheat breeding programs
- Germplasm will be developed that will combine tolerance to the major stresses that are encountered in sodic and dispersive soils and will focus on tolerance to salinity, high pH, aluminium (Al) toxicity and waterlogging.
Background
Alkaline soils (pH>8) cover 24% of Australia with 62 Mha occurring in south-east (SE) Australia. The chemical and physical properties of alkaline soils impose important constraints to yield yet their amelioration is difficult and consequently has been largley ignored. Subsoil constraints include salinity, sodicity, high soil strength and toxic concentrations of aluminium (Al) and boron (B) although a range of other factors, such as bicarbonate toxicity, nutrient deficiencies and water-logging have also been identified to occur in alkaline soils which have important implications for a number of current GRDC areas of investment, including water use effciency, crop nutrition, and germplasm development. Several soil constraints are estimated to adversely affect grain production (>$433M) through reduced infiltration of rainwater, poor crop establishment and limitations to root growth and functions which subsequently restrict the crops’ access to soil water and nutrients.
A key to improving crop productivity is to increase the root zone of crops to enable use of subsoil water located deep in the soil profile. In SE Australia, the root zone is often considered to be to a depth of 60cm to 100 cm, yet roots are potentially capable of much deeper growth if they are able to tolerate growing with subsoil constraints. A range of engineering and farm management solutions is available to ameliorate subsoil constraints (e.g. deep ripping, primer crops and subsoil manuring), but their cost and slow adoption mean that to maintain crop production in regions with subsoil constraints, a genetic approach, involving breeding cultivars with an enhanced ability to grow on affected land (Tavakkoli et al., 2012) in conjunction with the reclamation and management practices (Gill et al., 2008) is inevitable.
Historically, little has been known about genetic variation for subsoil constraints tolerance in Australian varieties, leading to the assumption that little variation exists. This limited knowledge is also due to the practical difficulties in measuring soil constraints under field conditions due to the dynamic nature of these constraints. However, successive GRDC co-funded projects on variety improvement for sodic, dispersive soils (UA00159) and management of subsoil constraints (DAN00206, DAV00149) is tackling these major methodological and knowledge gaps by developing viable managements options to improve productivity and use of plant breeding to develop better-adapted crops with improved physiological tolerance to subsoil constraints.
This paper provides results of a GRDC co-funded project (UA00159) aiming to increase wheat yields on sodic soils by pyramiding plant traits linked to sodicity tolerance into commercial wheat cultivars. Field trials testing cultivars of bread and durum wheat were conducted at 11 sites across Australia. The specific aim of these trials is to identify wheat cultivars with high grain yields in sodic soils and to determine the plant traits contributing to this yield.
Method
Validation of current germplasm
Identifying the right parental material is crucial for the success of the germplasm development program. While genetic variation for salinity tolerance, waterlogging, and Al tolerance has been described, this information has been derived from separate research programs with varying levels of field evaluation, much of which is regionally specific. A panel of up to 51 lines and check varieties of bread and durum wheat were grown in replicated trials in the Northern, Southern and Western GRDC regions on the targeted sodic soils. Experiments were conducted in three seasons. Local checks were included as well as a core set of lines recommended by project partners and breeders. This set of trials will provide a systematic comparison of the germplasm at the same sites and between different sodic soils.
In 2015 to 2017 three field trials were conducted in a grower’s paddock near Rand, NSW and included 44 wheat varieties in a fully randomised complete block design with four replicates. Rainfall for the growing season was 271mm (May to mid-November). The soil at the experimental site was a Red Sodosol with a starting mineral N content of 51kg/ha to a depth of 1.2m. The previous crop was canola which was burnt late prior to sowing. A Geonics EM38® instrument in vertical dipole mode, was used to measure the apparent electrical conductivity (ECa) of the soil. Based on the map of ECa, the most uniform area of each field was selected for the experiments.
The experiment was direct sown using Deep Blade System (DBS) tynes spaced at 250mm. At sowing 80kg mono-ammonium phosphate (MAP) (22kg phosphorus (P)/ha and 10kg nitrogen (N)/ha) was added to all plots. In-crop weed control was undertaken by applying the pre-emergents Sakura® (pyroxasulfone 850 g/kg) at 118 g/ha and Logran® (triasulfuron 750g/kg) at 35g/ha and was incorporated at sowing. Precautionary disease control was implemented, seed was treated with Hombre ® Ultra [Imidacloprid (360g/L) and Tebuconazole (12.5g/L)] at 200mL/100 kg and Prosaro® (Prothioconazole 210g/L and Tebuconazole 210g/L) was applied at 300mL/ha at DC 31. The experiment was harvested on 1 December. Grain protein and seed quality were estimated using near-infrared (NIR) (Foss Infratec 1241 Grain Analyzer) and Seed Imaging (SeedCount SC5000R), respectively.
At anthesis, about 50 of the youngest fully mature leaves (YML) were obtained randomly from each replicate plot of each genotype and then dried at 70◦C for 48 hours. Dried plant samples were digested in an acid mixture of nitric and perchloric acid and concentrations of ions were measured on Inductively Coupled Plasma (ICP).
Results and discussion
Soil constraints
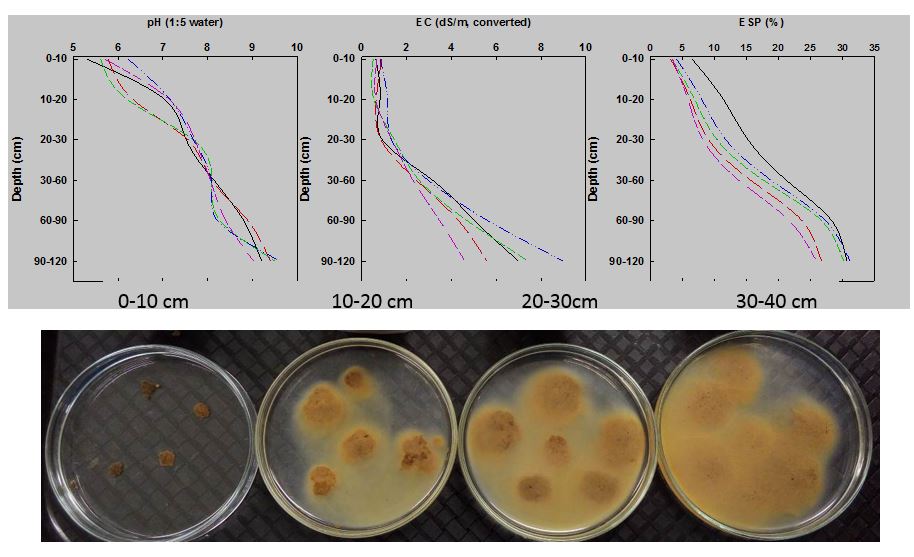
The depth-wise distribution of physicochemical soil constraints are given in Figure 1. The profile is characterised by the soil pH ranges from 5.1 to 9.1 with increasing sodicity (exchangeable sodium percentage (ESP) up to 30%) with depth. A dispersion test was performed on several aggregates and indicated significant dispersion in subsoil increasing with depth (Figure 1). A considerable amount of soil water below 60cm after harvest was also found which suggests limitations to root growth reduced the crop’s ability to access subsoil water (Rengasamy et al., 2016).
Figure 1(a). Selected soil characteristics of a sodic site in Rand (southern NSW). Various lines indicate multiple locations across the trial. The picture shows the assessment of soil dispersion at four different depths. The increasing levels of exchangeable sodium relative to calcium and/or magnesium in subsoil results in a decrease in soil structural stability and higher dispersion as shown in Figure 1(b).
When dispersion occurs (Figure 1b), the dispersed clay particles fill up the pores between soil particles and aggregates; and when the soil dries out, the dispersed clay blocks soil pores. This can restrict seedling emergence, water and air movement, and root penetration. Dispersed soils are generally hard-setting and may form a surface crust or concrete-like lump which can also result in waterlogging.
Variety evaluation
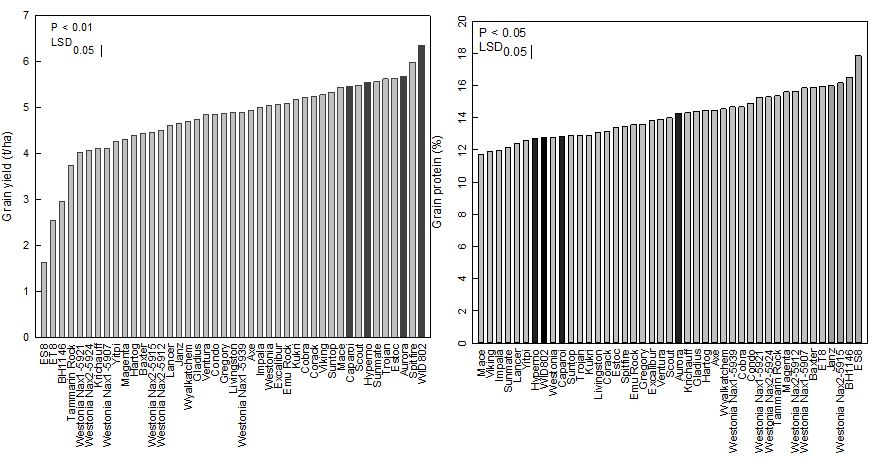
Significant genotypic variation occurred in grain yield and protein (Figure 2). The 2017, grain yield of the 44 genotypes (commercial lines) ranged from 3.74t/ha (Tammarin RockA) to 5.99t/ha (SpitfireA). Grain protein content varied from 11.7% to 17%.
Figure 2. Variation in grain yield and protein of 44 commercial and breeding lines of bread (grey bars) and durum (black bars) from the southern New South Wales (SNSW) site in 2017.
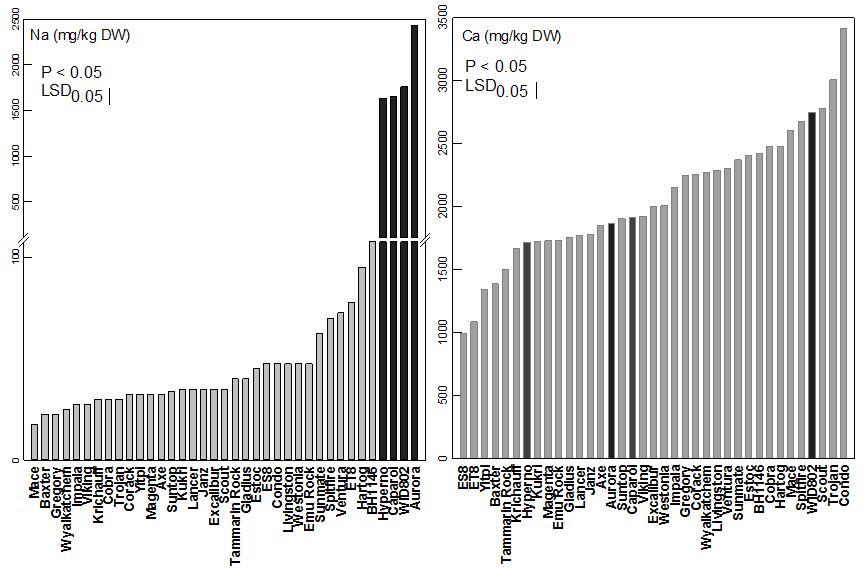
Sodium (Na) concentrations varied about six fold ranging from 17mg/kg DW to 108mg/kg dry weight (DW) (Figure 3). The Na concentration of durum lines (black bars) ranged from 1600mg/kg DW to 2427mg/kg DW. As well, the calcium (Ca) concentration varied about 3.4-fold ranging from 990mg/kg DW to 3400mg/kg DW. Grain yield was positively and significantly correlated with leaf Ca concentrations (r = 0.59, P < 0.05).
The aim of the current study was to investigate any potential genetic variation for performance on sodic soils with variable subsoil constraints in wheat genotypes either commercially available or with known adaptation to water-limitation, and to identify any potential selection criteria to select genotypes better adapted to these conditions. Considerable variation between genotypes was found, and some potential selection criteria were identified using measurements of yield, leaf element concentrations, in-season drone phenotyping and crop water use. Improving the grain yield of crops is always a major target in plant breeding. Therefore, the evaluation of final grain yield and growth parameters determining grain yield is a critical aspect of breeding programs.
Figure 3. Variation in leaf sodium (Na) and calcium (Ca) concentration of different wheat genotypes grown in sodosol soil at Rand, SNSW.
Conclusions
- Subsoil constraints limit grain yield of current wheat varieties in Australia.
- The preliminary results indicates a large genotypic variation exists among various varieties of bread and durum wheat which will be used to determine the plant traits contributing to yield.
- Evaluation of wheat varieties for tolerance to subsoil constraints using both field trials and greenhouse experiments is in-progress to identify suitable parental lines for future germplasm development.
References
Gill, J.S., Sale, P.W.G., Tang, C., 2008. Amelioration of dense sodic subsoil using organic amendments increases wheat yield more than using gypsum in a high rainfall zone of southern Australia. Field Crops Research 107, 265-275.
McDonald, G.K., Taylor, J.D., Verbyla, A., Kuchel, H., 2013. Assessing the importance of subsoil constraints to yield of wheat and its implications for yield improvement. Crop and Pasture Science 63, 1043-1065.
Rengasamy, P., Tavakkoli, E., McDonald, G.K., 2016. Exchangeable cations and clay dispersion: net dispersive charge, a new concept for dispersive soil. European Journal of Soil Science 67, 659-665.
Tavakkoli, E., Fatehi, F., Rengasamy, P., McDonald, G.K., 2012. A comparison of hydroponic and soil-based screening methods to identify salt tolerance in the field in barley. Journal of experimental botany 63, 3853-3867.
Acknowledgments
This research was undertaken as part of project UA00159 (co-funded by University of Adelaide, NSW DPI, DAFWA, DEDJTR, UQ, SARDI and GRDC). The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the authors would like to thank them for their continued support.
Thanks to Graeme Poile, Albert Oates, Vincent van der Rajit, and Yan Jia (Soils Unit, NSW DPI) for technical and intellectual inputs towards the NSW component of the project and Lucy Cunningham (University of Adelaide) for technical support. The authors also acknowledge Dr Julian Taylor for statistical support of the project.
Contact details
Ehsan Tavakkoli
NSW DPI, Wagga Wagga
02 69381992
Ehsan.tavakkoli@dpi.nsw.gov.au
GRDC Project Code: UA00159,
Was this page helpful?
YOUR FEEDBACK