Insects, resistance and control
Author: James L. Maino, Siobhan de Little, Lisa Kirkland, Elia Pirtle (cesar, University of Melbourne) Matthew Binns (University of Melbourne) Paul A. Umina (cesar, University of Melbourne) | Date: 13 Feb 2018
Take home messages
- Insecticide resistance issues continue to outpace novel control options.
- Redlegged earth mite (RLEM):
- Resistance in RLEM has been detected for the first time in eastern Australia.
- Synthetic pyrethroids (SPs) are completely ineffective against SP-resistant RLEM populations, while some efficacy remains for organophosphates (OPs) against OP-resistant RLEM populations.
- Aphids:
- Green peach aphid (GPA) has acquired low level resistance to neonicotinoids.
- Pirimicarb is now mostly ineffective against GPA due to resistance, but remains effective against other crop aphids, highlighting the importance of correct species identification.
- A variety of insecticide seed treatments have been shown to control Russian wheat aphid (RWA), with the length of protection differing between products.
- The implementation of recently published resistance management strategies (RMS) is vital to maximising the long-term viability of chemical options for pest management.
- Looking to the future:
- Growth in the use of neonicotinoids will likely see increased resistance issues and the disruption of beneficial insect services in Australia.
- Cutting edge forecasting tools are helping to identify patterns in insecticide resistance outbreaks.
ɸExtra technical comment by Protech Consulting Pty Ltd
Background
Insecticide resistance issues in broad-acre cropping continue to outpace the expansion of novel control options. In this paper, the latest findings on two major pest species that have developed resistance to key chemical groups, the redlegged earth mite (Halotydeus destructor, RLEM) and the green peach aphid (Myzus persicae, GPA) are discussed.
New research on the efficacy of seed treatments against Russian wheat aphid (Diuraphis noxia, RWA) is also presented.
The paper concludes by discussing the future risks of increased reliance on neonicotinoid insecticides and the application of forecasting approaches managing insecticide resistance.
Resistance in redlegged earth mites spreads to eastern Australia
The redlegged earth mite (Halotydeus destructor, RLEM) is an important pest of germinating crops and pastures across southern Australia. Four chemical sub-groups are registered to control RLEM in grain crops: organophosphates (OPs) (Group 1B); synthetic pyrethroids (SPs) (Group 3A); phenylpyrazoles (Group 2B); and neonicotinoids (Group 4A). The latter two are registered only for use as seed treatments (Umina et al. 2016).
After remaining confined to Western Australia (WA) for a decade, in 2016, pesticide resistance in RLEM was detected for the first time in eastern Australia (Maino, Binns and Umina, 2017). In WA, resistance to SPs is widespread, while OP resistance is comparatively more restricted (Figure 1). In 2016, following reports of a field control failure in the Upper South East district in South Australia (SA), resistance testing determined this SA population was resistant to SPs and OPs (Figure 2). In 2017, two additional SP resistant populations were confirmed on the Fleurieu Peninsula (approx. 30km apart from each other, and approx. 200km from the 2016 detection).
Figure 1. The current known distribution of H. destructor in Australia (adapted from Hill et al., 2012) shown as full circles, overlaid with the known distribution of SP and OP resistance across Australia at 2017.
All SP resistant populations tested to date have been found to possess a target site mutation on the para-sodium channel (Edwards et al. 2017). This mutation confers high level SP resistance (approx. 200 000 times the resistance of a susceptible population) leading to complete spray failures (Figure 2). In contrast, the mechanism conferring OP resistance has not yet been resolved, but resistance is comparatively less than SP resistance, such that OP efficacy will be reduced but not lost entirely.

Figure 2. Concentration-mortality curves for redlegged earth mite from a susceptible (DC01) and resistant (SA01) populations when exposed to a synthetic pyrethroid — bifenthrin (A) — and an organophosphate — omethoate (B) — after 8 hrs exposure. Vertical bars denote standard errors. Lines represent fitted values from fitted logistic regression models.
To increase management options for populations with dual resistance to OPs and SPs, trials run by the University of Melbourne and cesar are testing the impact of different management regimes on mite abundance and chemical tolerance in a dual-resistant population. Preliminary results have shown that both foliar chemical groups are largely ineffective on populations with SP and OP resistance, but that high rates of omethoate can still provide control in OP-resistant populations, though the long-term sustainability of this strategy is unlikely. A novel mode-of-action group was also tested as part of this trial and found to be highly effective at suppressing mite numbers, indicating no cross-resistance.
Green peach aphid acquires new resistances
Green peach aphid (GPA) is a widespread and damaging pest of canola and a range of pulse crops, causing damage by feeding and transmitting viruses. Five chemical subgroups are registered to control GPA in grain crops: carbamates (Group 1A); SPs (Group 3A); OPs (Group 1B); neonicotinoids (Group 4A); and sulfoxaflor (Group 4C). Paraffinic spray oils are also registered for suppression of GPA.
Together with CSIRO, cesar has been mapping the extent of insecticide resistance in GPA across Australia for the past few years. This ongoing resistance surveillance has continued to show high levels of resistance to carbamates and SPs that are widespread across Australia. Moderate levels of resistance to OPs have been observed in many populations, and there is evidence that resistance to neonicotinoids is spreading.
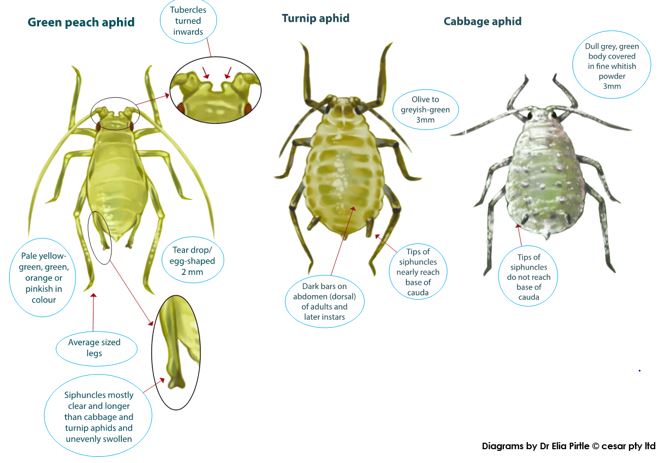
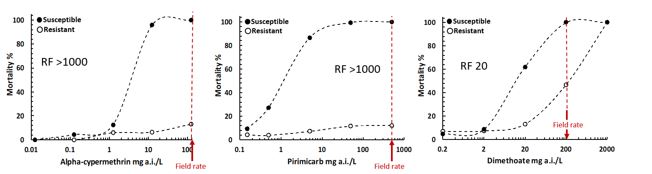
Despite widespread resistance to the aphid specific carbamate chemical pirimicarbɸ in GPA populations (Figure 4), this pesticide remains important to the control of other canola aphid species of similar appearance (e.g. cabbage aphid and turnip aphid). Thus, it is important to properly identify aphids before spray decisions are made. Figure 3 highlights some key features that can be used to distinguish GPA (with a hand lens) from other similar species found on canola. If a hand lens is unavailable, GPA will usually be found on the lowest, oldest leaves, typically in sparse family groups, while turnip aphid and cabbage aphid are more commonly found in large colonies on flower spikes.
ɸProducts containing pirimicarb are not registered for control of turnip aphid in canola. In commercial situations label specification must be adhered to at all times.
Neonicotinoid resistance conferred by enhanced expression of the P450 CYP6CY3 gene was discovered in Australian GPA populations in 2016 by cesar and CSIRO researchers. Laboratory bioassays revealed these aphids to be approx. 10 times more resistant to a topical application of a neonicotinoid compared to a susceptible population. However, overseas GPA are known to carry an R81T gene mutation of the nicotinic acetylcholine receptor that confers approx. 1000 times resistance to neonicotinoids resulting in field control failures well as cross-resistance with group 4C chemicals such as sulfoxaflor. Australian GPA populations may acquire this high level neonicotinoid resistance if neonicotinoid selection pressures remain high, or if there is an incursion of overseas GPA carrying the R81T mutation.
Figure 3. To assess the applicability of pirimicarb to other non-resistant aphid species of similar appearance, green peach aphid should be distinguished using diagnostic traits. If a hand lens is unavailable, green peach aphid will usually be found on lowest, oldest leaves, typically in sparse family groups, while turnip and cabbage aphid are more commonly found in large colonies on flower spikes.
Figure 4. Sensitivity of a typical Australian susceptible and resistant green peach aphid population to the synthetic pyrethroid, alpha-cypermethrin (left panel), the carbamate, pirimicarb (middle panel) and the organophosphate, dimethoate (right panel). RF = Resistance Factor.
Resistance management strategies
With resistance evolution continuing to outpace the discovery of new chemistries with novel modes of action, resistance management strategies (RMS) are more than ever essential to maintain the viability of pest control tools.
RMS for major grains pests have been made available through the National Insecticide Resistance Management (NIRM) working group of the Grains Pest Advisory Committee, a GRDC funded project, which provides strategic advice to GRDC on pest issues. Across these strategies, there are both general and pest-specific practices that can help maintain the viability of chemistries into the future.
General RMS strategies include:
- If applying multiple insecticides within a season, rotate chemistry mode of action.
- Utilisation of non-chemical control options that suppress pest populations.
- Using economic spray thresholds to guide chemical applications.
- Using selective chemicals, if chemical application deemed necessary, in place of broad-spectrum options.
- if using broad spectrum chemicals, consider the secondary impacts to non-target pests and beneficials.
- Compliance with all directions for use on product labels and ensuring proper application coverage.
RMS strategies specific to GPA include:
- Managing the green bridge (in particular, the control of brassica weeds and volunteer crops) on which GPA may persist through summer.
- Stubble retention to decrease visual contrast between seedlings and soil (landing cue for GPA).
RMS strategies specific to RLEM include:
- Control of spring populations immediately before the production of over-summering (diapause) eggs through cultural control (grazing, broadleaf weed removal), or a Timerite® spray (if required) to reduce pest pressure at crop emergence/RLEM hatching the following autumn.
Testing control methods for Russian wheat aphid
Russian wheat aphid (Diuraphis noxia, RWA) was first detected in Australia in 2016. The host range of RWA includes more than 140 species of cultivated and wild plants within the family Gramineae (grasses). These include wheat, barley, triticale, rye, oats, pasture grasses and wild genera including Poa, Bromus, Hordeum, Lolium, Phalaris and others. Wheat and barley are most susceptible, while triticale, rye and oats are less susceptible.
Unlike other cereal aphids that damage plants by removing nutrients, RWA also injects salivary toxins during feeding that cause rapid, systemic phytotoxic effects on plants, resulting in acute plant symptoms and potentially significant yield losses. Even a few aphids can cause plant damage symptoms to appear as early as 7 days after infestation. These include:
- white and purple longitudinal streaks on leaves
- curled, rolled or hollow tube leaves
- stunted growth or flattened appearance
- discolored leaves
- hooked-shaped head growth from awns trapped in curling flag leaf
- bleached heads
Insecticide seed dressingsɸ can be effective to combat RWA infestations in establishing cereal crops. cesar have tested the relative efficacy and length of activity of various insecticide seed dressings in wheat against RWA, and compared this with another important cereal aphid pest, the oat aphid (Rhopalosiphum padi).
ɸNone currently registered for use in Australia, but their use is permitted under the following permits: PER81133, PER82304 and PER83140.
All seed dressings tested provided effective aphid control up to five weeks after emergence, with higher rates generally providing several weeks extra protection over lower rates of the same product. Oat aphids generally persisted and reproduced on wheat at an earlier time-point than RWA, suggesting that RWA is less tolerant to the insecticide seed dressings tested. This suggests that management of cereal aphids in Australia using insecticide seed dressings is likely to achieve similar, if not better, control of RWA as oat aphid.
Balancing the scales of neonicotinoid seed treatment use
Neonicotinoids are currently the most used insecticide group globally. This over-reliance may be explained by the increased resistance issues surrounding older chemistries like the OPs and SPs. Also contributing to this trend is the convenience of neonicotinoids, in particular, as seed treatments, which are applied at the time of sowing at no extra application cost.
Despite the advantages of neonicotinoid seed treatments, their indiscriminate usage as commonly seen, carries some important costs. Continued wide-scale use of neonicotinoid seed treatments will select for resistance, as is currently being seen in GPA in Australia (de Little et al. 2017). Overseas, where neonicotinoids have been used for longer and more extensively, more cases of resistance have been documented (Sparks and Nauen, 2015). In addition to resistance concerns, widespread neonicotinoid use is likely to impair ecosystem services provided by some beneficial invertebrate and microbial communities, as has been shown in international studies. Industry stewardship and good resistance management are paramount to ensuring neonicotinoid usage is balanced against these issues, and remains a long-term viable control option for grains pests.
Before making a management decision, the question should be asked, is a neonicotinoid seed treatment warranted in this paddock, in this year?
- Wherever possible, assess the risk of damaging pest infestations (or virus risk), based on the prior paddock and seasonal history. In the case of RLEM, for example, a high-risk situation would be indicated by: (i) canola or lucerne to be sown, (ii) high mite numbers the previous year, and (iii) no Timerite® spray the previous spring.
- Unless the pest risk is deemed high, avoid using neonicotinoid seed treatments in consecutive years, preferably no more than one in three years in any given paddock.
With seed treatments, which are not applied in response to immediate pest pressure, the challenge, of course, is the ability to accurately forecast the timing and severity of pest (and virus) occurrences well ahead of time. Predictive tools may provide useful information here, but are currently not being used for such purposes, or simply do not exist for a particular species of interest.
Forecasting future resistance issues
To bring further focus to the resources directed to resistance management, researchers from cesar and the University of Melbourne have applied modern forecasting approaches to identify spatial relationships in the evolution of resistance. This novel approach synthesised large data sets on resistance, land usage, and environmental factors, and found that resistance in RLEM is related to chemical pressure (average number of chemicals used annually), but more surprisingly is also more likely to develop in regions with particular climatic properties (Figure 5). The study highlighted risks in eastern Australia before the recent detection of resistance in SA, and will be used to guide resistance management in the future.

Figure 5. Predicted pyrethroid resistance risk (probability) for RLEM adapted from Maino et al. (in press). Known resistant populations used to calibrate the model (open circles) as well as newly detected populations (open triangles) are overlaid.
Useful resources
Resistence Management strategy for the Green Peach Aphid in Australian Grains
RLEM-Resistance-strategy-South
References
Edwards, O. R. et al. (2017). ‘A genomic approach to identify and monitor a novel pyrethroid resistance mutation in the redlegged earth mite, Halotydeus destructor’, Pesticide Biochemistry and Physiology, p. doi: https://doi.org/10.1016/j.pestbp.2017.12.002.
Hill, M. P. et al. (2012). ‘Understanding niche shifts: Using current and historical data to model the invasive redlegged earth mite, Halotydeus destructor’, Diversity and Distributions, 18(2), pp. 191–203. doi: 10.1111/j.1472-4642.2011.00844.x.
de Little, S. C. et al. (2017). ‘Discovery of metabolic resistance to neonicotinoids in green peach aphids (Myzus persicae) in Australia’, Pest Management Science, 73(8), pp. 1611–1617. doi: 10.1002/ps.4495.
Maino, J. L., Binns, M. and Umina, P. A. (2017). ‘No longer a west-side story – pesticide resistance discovered in the eastern range of a major Australian crop pest, Halotydeus destructor (Acari: Penthaleidae)’, Crop and Pasture Science.
Maino, J. L., Umina, P. A. and Hoffmann, A. A. (no date). ‘Climate contributes to the evolution of pesticide resistance’, Global Ecology and Biogeography, p. n/a--n/a. doi: 10.1111/geb.12692.
Sparks, T. C. and Nauen, R. (2015). ‘IRAC: Mode of action classification and insecticide resistance management’, Pesticide Biochemistry and Physiology. The Authors, 121, pp. 122–128. doi: 10.1016/j.pestbp.2014.11.014.
Umina, P. A. et al. (2016). ‘Science behind the resistance management strategy for the redlegged earth mite in Australian grains and pasture’.
Acknowledgements
The research presented here is made possible by the significant contributions of growers through both trial cooperation and the support of the Grains Research and Development Corporation. The authors would like to thank them for their continued support.
We would also like to acknowledge other collaborators including Julia Severi (cesar), Ary Hoffmann (University of Melbourne), Owain Edwards (CSIRO), Jenny Reidy-Crofts (CSIRO), Svetlana Micic (DAFWA), and Alan Lord (DAFWA). The authors also acknowledge the assistance of Ken McKee and David Landmeter (Syngenta Australia), Shane Trainer (Bayer Crop Science) and Colin Edmondson (Advanta Seeds).
Contact details
James Maino
cesar Pty Ltd, 293 Royal Parade, Parkville VIC
03 9349 4723
jmaino@cesaraustralia.com
Paul Umina
cesar Pty Ltd, 293 Royal Parade, Parkville VIC
03 9349 4723
pumina@cesaraustralia.com
@PestFactscesar
Was this page helpful?
YOUR FEEDBACK