Sowthistle biology – management & resistance status
Author: Paul McIntosh (Australian Herbicide Resistance Initiative) | Date: 04 Dec 2018
Take home message
- Sowthistle (Sonchus oleraceus) can produce up to 200,000 seeds per well grown adult plant.
- Seed has no or minimal dormancy
- Zero or minimum till favours all year round germination of sowthistle
- Use all methods to stop seed set and rundown seed bank reserves of this predominate surface germinator
- Each seed has a feathery pappus to aid more distant dispersal by wind
Survey of glyphosate resistance in Queensland and New South Wales
Sowthistle is one of the most widespread weeds in the northern cropping region and has the ability to germinate at most times during the year.
In approximately 600 paddock surveys in Qld and NSW;
- Winter 2016/17- 202 paddocks had sowthistle present
- Summer 2017/18- 11 paddocks had sowthistle present
Distribution
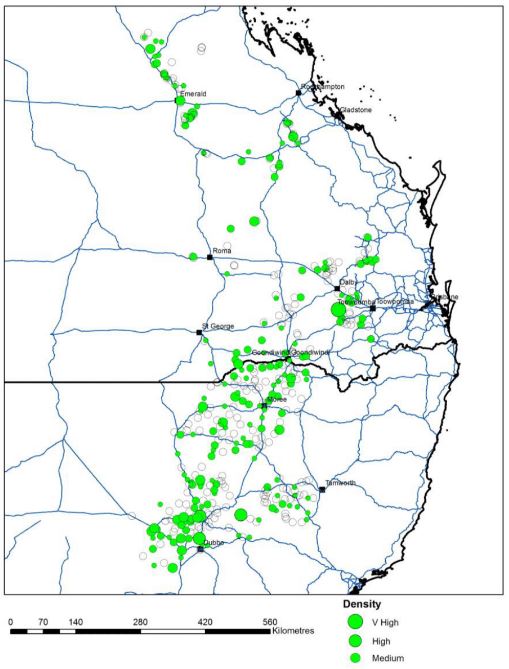
Annual sowthistle is widely distributed in Australia. Virtual herbarium records indicate the presence of this weed in all Australian states and territories. (Figure 1).

Figure 1. Distribution of annual sowthistle across Australia
Figure 2. Sowthistle distribution in the northern grain cropping region (Source: Broster J (2018), Charles Sturt University and Jalaludin A, Queensland Department of Agriculture and Fisheries, (2018)).
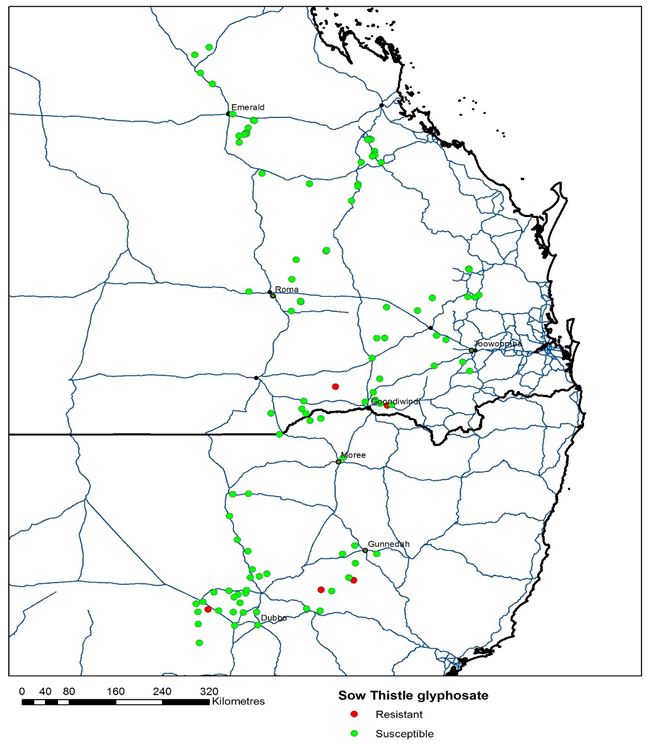
The recent development of populations that are resistant to glyphosate has the potential to greatly complicate and increase cost associated with management of this weed – particularly in the fallow.
Figure 3. Map of glyphosate resistant and susceptible sowthistle (Sonchus oleraceus) populations across northern grain cropping region. All populations remained sensitive to 2,4-D amine.
(Source: Broster J (2018), Charles Sturt University and Jalaludin A, Queensland Department of Agriculture and Fisheries, (2018)).
Biology and management of annual sowthistle (Sonchus oleraceous L.)
Figure 4. Sowthistle (photo credit: State of Queensland 2015, Department of Agriculture & Fisheries)
Salient features
- Annual sowthistle is a broad leaf weed and member of the ‘Asteraceae’ family
- Abundant seed production, wind dispersal, lack of seed dormancy and ability to germinate under varying light and temperature conditions, soil pH and salinity and its preference to germinate at or very close to the soil surface, can lead to a further increase in the predominance of this weed – particularly in minimum and zero tillage crop systems
- Management should focus on diversifying the type of control options by including competitive crops in rotation that also allow the use of non-glyphosate based control strategies
- Annual sowthistle germination, biomass production and total weed seed production rates are significantly reduced by crop competition. Crop competition can be enhanced by selecting more competitive crop types, using narrow row spacing and high seeding rate
- In a fallow environment, sowthistle has the capacity to deplete substantial soil moisture
- Annual sowthistle naturally exhibits a level of glyphosate tolerance. This tolerance has evolved with populations now confirmed as resistant to glyphosate. Risk assessment studies indicate that this weed has the potential to rapidly evolve resistance to glyphosate
- Sowthistle is a small seeded surface germinating weed which therefore struggles to germinate from depth. As such, tillage based systems where a substantial amount of weed seed is placed at depth reduce weed emergence
- Control of sowthistle products with residual products can be highly effective due to the low levels of seed dormancy and persistence
Major morphological features
Annual sowthistle develops bigger basal leaves (up to 30 cm). New leaves added as the plant grows are progressively smaller. Leaf development is alternate with leaves clasped around the stem. Plants can grow up to 1.5 m high. Stems are hollow and will ooze a white sap when broken. Flowers are yellow (dandelion like) and, when mature, develop into a small tuft of white flower head suited to wind dispersal (Figure 5). The yellow flowers and seed head closely resemble another species called ‘prickly sowthistle’ (Sonchus asper L.). As the name indicates, prickly sowthistle has spiny leaves and can be easily distinguished from annual sowthistle (Figure 6).
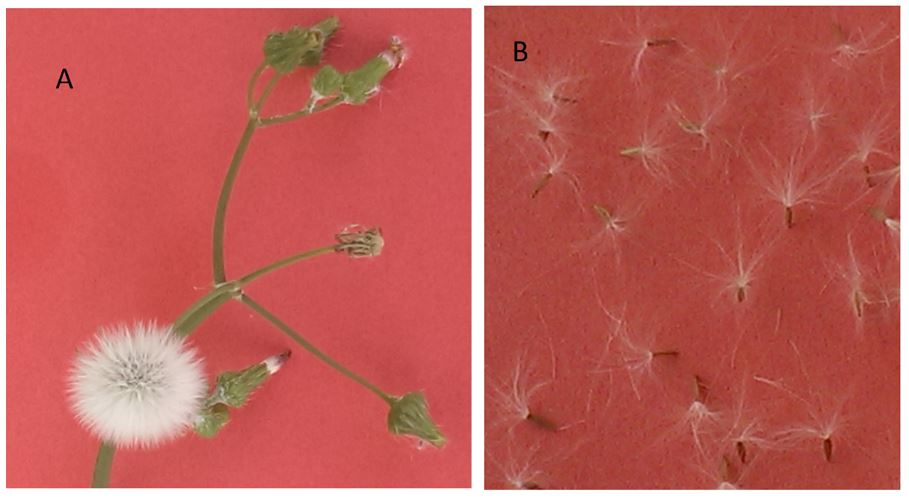
Figure 5. Annual sowthistle with white flower head and individual seeds with white pappus (Source: B. Chauhan)
Figure 6. Prickly sowthistle (A) and annual sowthistle (B) bearing flower heads (Source: B. Chauhan)
Germination
Previous studies from South Australia and Queensland indicated the potential of this weed to emerge under a broad range of light and temperature conditions. Darkness inhibited germination compared to an alternating light and dark environment, however 40% germination was still achieved in the dark environments.
Annual sowthistle was also shown to germinate under a broad range of soil pH, salinity conditions and water stress environments, although germination was substantially higher under non-water stressed environments where surface soil water was close to saturation.
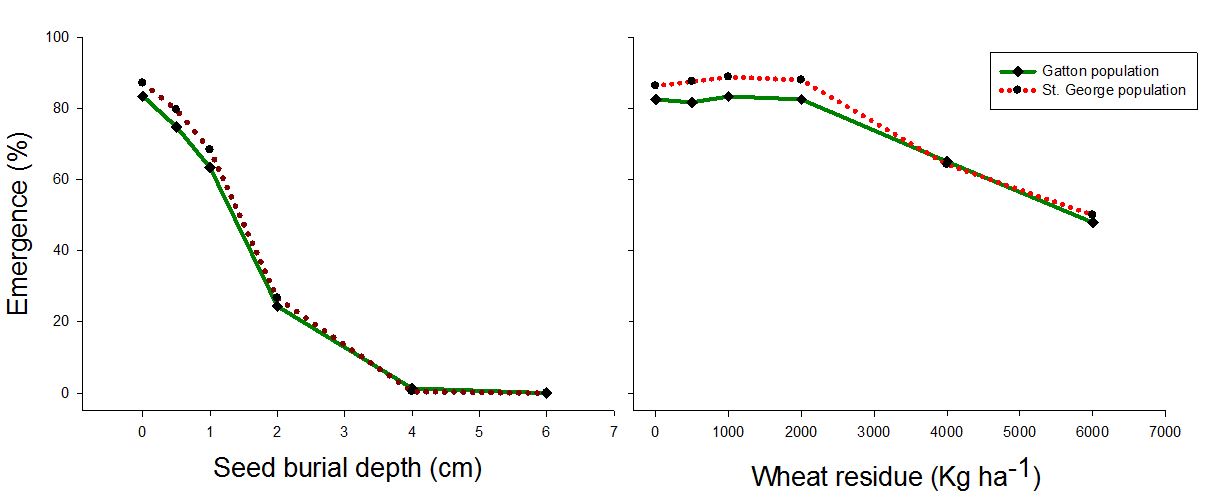
Recent studies investigating the effect of seed burial depth and the impact of stubble residue on germination have shown the following results.
Figure 7. Effect of seed burial and residue cover on the emergence pattern of two populations of annual sowthistle sourced from St George and Dalby areas of Queensland (Manalil and Chauhan, GRDC UA000156)
Salient findings
- Freshly collected seeds readily germinated in water (more than 85%) indicating lack of dormancy in freshly harvested seeds
- Annual sowthistle couldgerminate under a broad range of temperatures (5/15, 10/20, 15/25 and 20/30 degrees Celsius day/night temperatures), germination was the lowest at 5/15 oC. Notably, germination was more rapid at 15/25 and 20/30 C day/night temperatures than at 10/20 oC. This indicates the adaptability of populations from Queensland to warmer environments
- Continuously dark conditions inhibited germination
- Significant reduction in germination was observed in studies when residue cover was increased to 4000 kg/ha compared to no-residue cover (Figure 7)
- Annual sowthistle germinated over a broad range of soil pH (4 to 10), with a level of germination observed at osmotic potential as high as -8 MPa and at salinity level of 200 Mm
- Seed germination decreased with increasing depths. Zero germination was observed at 4-6 cm depth (Figure 7)
Seed persistence at different depths
In work conducted by Manalil and Chauhan in 2015-17, seed persistence was evaluated for a period of 18 months at 0 cm, 2 cm and 10 cm depths on two populations from St George and Dalby. Seeds were exhumed at different times (0, 3, 6, 12, 18 months after burial) and examined in the laboratory for their germination.
Salient findings
- At 3 months, there was 62% and 59% lab germination from seeds placed on the surface layer for the Dalby and St George populations, respectively. However, there was a rapid decline in germination from the surface layer over time. At 6 months, germination declined significantly to less than 11% germination from the surface layer. At 18 months, seeds at the surface layer were completely germinated (in the field) or decayed.
- There was around 80% lab germination from seeds buried at 2 cm depth at 3 months. There was significant reduction in germination at this layer over time. At 12 and 18 months, lab germination at this layer declined to around 30% and 13%, respectively.
- For seeds that were buried at 10 cm, the pattern of lab germination was similar to the 2 cm depth, although germination was slightly lower than the 2 cm layer. There was around 60% lab germination observed for seeds buried at this layer at 3 months. Germination declined significantly over time with less than 17% and 8% germination at 12 and 18 months, respectively.
Results of burial depth and seed persistence studies indicated the possibility to include tillage (aiming for seed burial) as an option for the management of this weed. This is because there was significant reduction in the level of emergence from seeds buried at layers deeper than 2 cm (less than 25%) and the seed depletion over time as indicated by the seed persistence study. The results justify grower experience of successful management of this weed using tillage.
Competitiveness of annual sowthistle
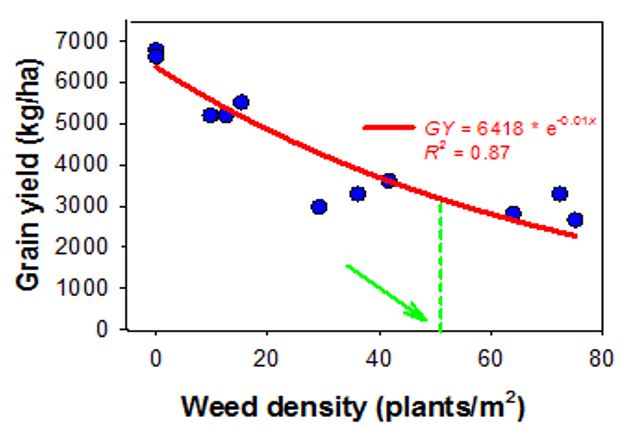
A study at Gatton Queensland explored the competitiveness of annual sowthistle in an irrigated wheat crop. The density for sowthistle was 0, 13, 36 and 70 plants/m2 for nil, low, medium and high-weed density plots, respectively (Figure 8). The corresponding yield loss was 20%, 50% and 56% at low, medium and high densities of sowthistle, respectively. The analysis indicated that the weed density of 51 plants/m2 annual sowthistle could cause a yield reduction of 50% under the tested environment.
Figure 8. An exponential regression model showing the relationship of wheat yield and weed density (at the anthesis time) of annual sowthistle. The green arrow shows the weed density (plants/m2) to reduce 50% of the maximum grain yield (Manalil and Chauhan, GRDC UA000156)
Management of annual sowthistle
- Crops vary in their competitive potential to supress annual sowthistle. Barley, with its capacity to generally attain canopy closure earlier than wheat, would supress weeds better than wheat
- Increasing wheat seeding rate in a good year would assist the crop to out-compete weeds and minimise yield losses from uncontrolled weeds. Similarly, narrow crop rows (e.g. 25 cm for cereals) could suppress annual sowthistle more than a crop sown on 50 cm rows
- Tillage can be used to control the emerging weeds and bury the seeds. It is always advisable to allow the seeds on surface to emerge before tillage as that would deplete a substantial portion of seeds in the soil surface
- Weed blowouts in fallows often follow weeds that survive control in winter crops. For this reason, sound management and control strategies in winter crops is a critical control strategy
- Weed management in fallow is also very important, as this weed can establish in spring by exploiting the nutrients and resources of the winter crop and use fallow as a breeding place (Figure 9)
Figure 9. Annual sowthistle establishment in a fallow at Gatton (photograph taken
by B. Chauhan on 27th August 2017).
Weed control options
Annual sowthistle has already evolved resistance to glyphosate (Group M), chlorsulfuron (Group B), and 2,4-D (Group I) in Australia. This necessitates implementing a diverse approach to the management of this weed species. Growers are recommended to implement a range of strategies as identified in the WeedSmart “The Big 6”.
- Rotate crops and pasture
- Double knock
- Mix and rotate herbicides
- Stop weed seed set
- Crop competition
- Harvest weed seed control
Herbicide options
A range of products are available for the control of annual sowthistle but care should be taken to maximise and preserve their efficacy. All products should be applied at the correct growth stage, increasing application water volume as plants size increases and avoiding spraying weeds during period of acute water and heat stress. Herbicide options for the control of sowthistle include:
- In fallow situations apply Group M (glyphosate), Group L (paraquat, diquat), Group N (glufosinate), Group G, and Group Q (Amitrole) herbicides, standalone or in mixtures as per label directions.
A double knock approach may help to minimise the impact annual sowthistle has in the fallow. Ensure glyphosate standalone or in mixtures is followed by Spray.Seed®, paraquat or other suitable 2nd knock herbicides such as Group G (saflufenacil).
Using camera sprayer technology in fallows with labelled or permitted herbicides may also allow for the precision application of herbicides so the costs of fallow management can be minimised.
- Pre-emergent herbicide options can be used prior to the planting including Group C, Group G (flumioxazin) and Group H (isoflutole) herbicides providing residual control of emerging sow thistle plants as per label directions.
- Use of registered products containing herbicide Groups C, F, H and I herbicides can be useful to help manage weeds in crop prior to weed seed set as per label directions.
- At crop maturity, use of herbicides in Groups M, L and G (saflufenacil) can be used in various crops as desiccants or spray topping of late weeds as per label directions.
Note: All products must be applied in accordance with label “Directions for Use” as published by the APVMA.
Sources
Chauhan B.S., Gill G. and Preston C. 2006. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus L.) in southern Australia. Weed Science 54: 854-860.
Online resource DAF Queensland. Preventing herbicide resistance in 'at risk' weeds. Accessed on 26/8/2017.
Online resource GRDC. Integrated weed management in Australian cropping system. Accessed on 25/8/17.
On line resource. Department of Agriculture, Queensland. Management of common sowthistle (fact sheet). Contributors: Michael Widderick and Steve Walker.
Online resource. Northern IWM factsheet. Common sowthistle, Sonchus oleraceous L. Ecology and Management. Contributors: Chauhan B.S. Widderick, M, Werth J, Cook T. Department of Agriculture and Fisheries, Queensland, (2015).
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers and agronomists through both trial cooperation and the support of the GRDC, Queensland Government, Charles Sturt University and The University of Sydney. The author would like to thank them for their continued support.
Contact details
Paul McIntosh
Australian Herbicide Resistance Initiative
Address: 7 Berghofer Drive, Highfields Qld 4352
Mb: 0429 566 198
Email: paul@pulseaus.com.au
® Registered trademark
Was this page helpful?
YOUR FEEDBACK