Ascochyta blight in intensive cropping of pulses
Take home messages
- PBA Hurricane XT is now rated moderately resistant – moderately susceptible (MRMS) for foliar ascochyta blight in South Australia (SA) due to shifts in the pathogen population, most likely in response to intensive cropping of this cultivar.
- The newly released cultivar PBA Hallmark XT is also rated MRMS to ascochyta blight and as this cultivar has the same source of resistance as PBA Hurricane XT growers must be vigilant in monitoring for signs of disease.
- The lentil cultivar Nipper was infected by a lower percentage of recently collected isolates in controlled environment studies compared to previous years suggesting that cultivar rotation can manipulate the pathogen population.
- Faba bean cultivars have not changed their resistance status to ascochyta blight since 2015. Screening of recent isolates of Ascochyta fabae confirmed that Farah remains susceptible to all strains of ascochyta blight, PBA Rana and PBA Zahra are rated, while PBA Samira and Nura remain resistant.
- Previous reports of higher than expected levels of ascochyta blight in commercial crops of PBA Samira faba beans are likely due to outcrossed seed being kept from the previous year for planting. Tests using A. fabae isolates on pure PBA Samira seed showed a resistant response to current ascochyta blight pathogen strains.
- Chickpea cultivars in the southern region are now all moderately susceptible or susceptible to ascochyta blight, requiring a thiram-based seed dressing and multiple foliar fungicide applications.
- Growers are encouraged to review their integrated disease management (IDM) strategies including cultivar selections for pulses in their farming system. To help protect the industry from loss of disease resistance, implement a 3-4 year break between crops of the same type, revise cultivar selections and avoid sowing in paddock(s) in close proximity to previous years’ crops. Growers should monitor crops for signs of disease and ensure fungicide applications are applied ahead of rain fronts.
Pathogenicity testing indicates shifts in the A. lentis pathogen population in South Australia
Forty isolates of Ascochyta lentis collected in 2017 from lentil field trials and commercial crops (36 from SA, four from Victoria (VIC) were tested in controlled environment conditions in 2018 on a differential host set that included the Australian commercial cultivars Nipper and PBA Hurricane XT (Table 1). Half of the isolates tested were capable of causing lesions on PBA Hurricane XT and almost a third (32.5%) were capable of infecting the resistant line Indianhead which is the presumed source of resistance in PBA Hurricane XT. This is an increase of 22.5% and 27.5% in the number of isolates capable of infecting PBA Hurricane XT and Indianhead respectively, compared to testing of 2015 isolates conducted in 2016 (Table 2).
The A. lentis pathogen population is naturally variable and aggressive forms are selected over time in intensive cropping systems. This has been seen with the intensive cropping of PBA Hurricane XT on the Yorke Peninsula and in the Lower North in SA where crops have developed moderately susceptible leaf lesions during 2015-2018. In 2018, PBA Hurricane XT was the dominant cultivar representing up to 90% of the lentils cropped in SA (pers. comm. Sam Holmes, Central Ag Solutions). The downgrading of the foliar rating of PBA Hurricane XT to MRMS in SA reveals that the longevity of this source of resistance is threatened by the current cropping practices in these regions. The newly released lentil cultivar PBA Hallmark XT is also rated MRMS to foliar ascochyta blight and appears to have the same source of resistance as PBA Hurricane XT. Therefore, the risk of ascochyta blight developing in PBA Hallmark XT is increased if this cultivar is sown into, or near, paddocks that have recently grown PBA Hurricane XT. Both cultivars may require podding sprays if disease is present during the growing season. Growers are urged to diversify their cultivar and crop rotations and monitor crops regularly for symptoms of disease.
In VIC, PBA Hurricane XT has retained its foliar moderately resistant (MR) rating for ascochyta blight as the shift in the pathogen population towards more aggressive isolates on PBA Hurricane XT has not been observed. Compared to SA, growers in VIC are not as reliant on a single cultivar such as PBA Hurricane XT in their farming systems, have longer rotations between growing lentil crops and the climate is generally colder and drier (pers. comm. Jason Brand, Agriculture Victoria). However, only four of the 40 A. lentis isolates tested in 2018 were collected from VIC lentil crops. Thus, the ability of isolates to infect PBA Hurricane XT present in the VIC population may have been underrepresented in the most recent testing. A. lentis requires cool weather and humidity for infection to occur where frequent rainfall events and a high load of inoculum in the form of infested lentil stubble can promote the likelihood of disease epidemics. Thus in VIC, the lower inoculum load coupled with suboptimal climatic conditions means that disease pressure for development of ascochyta blight in lentil crops is lower than that seen in SA.
Interestingly, the shift in the pathogen population has also seen a decrease in the number of A. lentis isolates capable of infecting the lentil cultivar Nipper, rated MRMS to foliar ascochyta blight, which is no longer widely adopted by industry. Nipper lost effective resistance to ascochyta blight in 2011, just four years after commercialisation and at the peak of its popularity comprised 30% of lentil cropping in SA (Davidson et al 2016). Less than 40% of the isolates collected in 2017 and tested in 2018 were capable of infecting Nipper, whereas two years ago all the isolates tested were capable of infecting the cultivar. This suggests that cultivar rotation has a strong effect on aggressiveness in the pathogen population structure by selecting virulent isolates to that cultivar. Growers are asked to consider whether cultivar rotation is possible in their farming system.
Table 1. Forty Ascochyta lentis isolates collected in 2017 were inoculated onto a lentil host differential set in controlled environment conditions in 2018. Entries in the table are the number of isolates per category.
Test reaction | Cumra (susceptible check) | Nipper | PBA Hurricane XT | ILL7537 (resistant line) | Indianhead (resistant line) |
|---|---|---|---|---|---|
R | 3 | 25 | 20 | 40 | 27 |
MR | 12 | 4 | 3 | 0 | 8 |
MRMS | 16 | 4 | 5 | 0 | 5 |
MS | 6 | 7 | 9 | 0 | 0 |
S | 3 | 0 | 3 | 0 | 0 |
Note: R = resistant, MR=moderately resistant, MRMS = moderately resistant-moderately susceptible, MS = moderately susceptible; S = susceptible
Table 2. Percent change of Ascochyta lentis isolates capable of infecting lentil host differential set collected from lentil field trials and commercial crops in 2015 and 2017 then tested in 2016 and 2018 respectively in controlled environment conditions. Entries in the table are the number of isolates capable of causing ascochyta blight lesions and the percent change (increase or decrease) observed in testing conducted in 2018 compared to 2016. Lesions can range from S to MR reactions as identified in Table 1.
Lentil cultivar/line | Year tested | % change | |
|---|---|---|---|
| 2016 | 2018 | ||
Cumra (susceptible check) | 40 | 37 | ↓ 7.5% |
NipperA | 40 | 15 | ↓ 62.5% |
PBA Hurricane XTA | 11 | 20 | ↑ 22.5% |
ILL7537 (resistant line) | 4 | 0 | ↓ 10% |
Indianhead (resistant line) | 2 | 13 | ↑ 27.5% |
Stubble from fungicide-treated lentil crops can harbour A. lentis spores that can infect resistant lentil cultivars
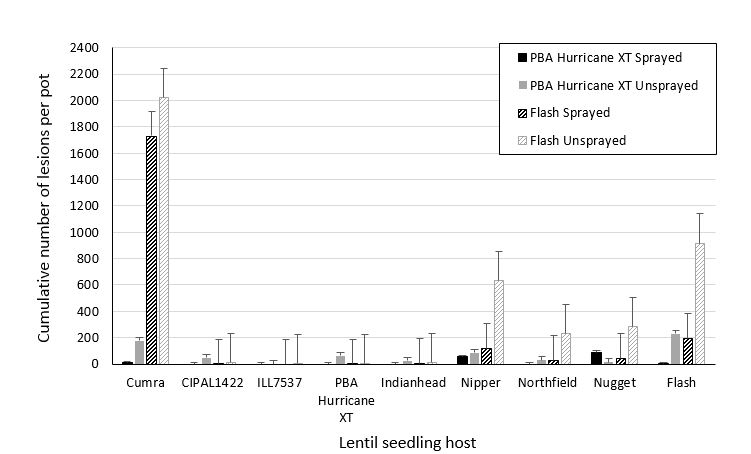
Lentil stubble from a fungicide field trial at Maitland SA, infested with A. lentis, was collected in December 2017 from plots of treated and untreated PBA Hurricane XT and PBA Flash. The stubble was placed into separate mesh bags according to cultivar and treatment type and left to weather outside on the SARDI Waite campus for three months. At the beginning of May individual pots of nine lentil cultivars were sown and placed around the mesh bags to capture spore splash from the stubble. Once seedlings were three weeks old, the number of lesions that formed on plants in each pot were counted and collected every week for 15 weeks (Figure 1). Lentil cultivars included those available commercially such as PBA Hurricane XT, PBA Hallmark XT (tested as CIPAL1422), Nipper, Northfield, Nugget, Flash, the two resistant lines ILL7537 and Indianhead, and the susceptible check Cumra.
Reassuringly, fewer lesions formed on lentil seedlings surrounding stubble from fungicide treated plots compared to untreated stubble. However A. lentis isolates exist in SA that can overcome the major sources of resistance. While there was a very low rate of infection (number of lesions) on PBA Hallmark XT, PBA Hurricane XT and Indianhead (resistant line, and presumed source of resistance in both the XT lentil lines used here), high selection pressure brought about by high cropping intensity of both of these cultivars could result in the selection of more aggressive forms of the pathogen, and loss of effective resistance, as was seen with the Nipper cultivar (Davidson et al 2016). Furthermore, as there was a significant number of lesions able to develop on a range of lentil hosts in the presence of stubble from fungicide treated plots, and considering the amount of stubble and spore production possible over an entire paddock, stubble from fungicide treated crops is still a major source of inoculum for future crops.
Figure 1. Cumulative number of ascochyta blight lesions over 15 weeks assessed on five lentil seedlings per pot adjacent to naturally infested lentil stubble from Maitland fungicide field trial. Stubble was from PBA Hurricane XT and Flash plots that had either been sprayed or not sprayed with Chlorothalonil every three weeks during the growing season. Vertical bars represent standard errors of the mean. CIPAL1422 = PBA Hallmark XT.
Resistance to ascochyta blight in current commercial faba bean cultivars remains stable
Faba beans are open pollinated which can lead to mixing of genetic material from grower retained seed. These genetic mixtures can lead to perceived changes in the resistance of a cultivar. The breeding program retains pure (‘ascochyta resistant’ or AR) lines for crossing and genetic studies, and these lines have been used to test the stability of ascochyta blight resistance in the commercially available cultivars Farah, PBA Samira and Nura. Forty isolates of Ascochyta fabae collected in 2017 from faba bean field trials and commercial crops (29 from SA, 10 from VIC, one from New South Wales (NSW) were tested in controlled environment conditions on a differential host set that included the commercial cultivars PBA Rana and PBA Zahra.
Faba bean cultivars have not changed their reactions to ascochyta blight since 2015 (Table 3) and screening of the isolates confirmed two pathotypes are still observed. Pathotype 1 is widely distributed in the southern region, and pathotype 2 which was identified in the mid-north of SA in 2013 is becoming established on the Yorke Peninsula and in the South East growing regions of SA and in VIC. In the presences of pathotype 2 cultivar Farah is moderately susceptible to ascochyta blight while PBA Rana and PBA Zahra are partially compromised. PBA Samira and Nura remain resistant to all pathotypes of A. fabae present in the pathogen population. For the newly released cultivars, the Group B herbicide tolerant PBA Bendoc is resistant to both pathotypes of A. fabae while PBA Marne, like PBA Rana and PBA Zahra, is only resistant to pathotype 1. A comprehensive fungicide strategy is required to control ascochyta blight in Farah and strategic fungicide applications at podding are required for PBA Rana and PBA Zahra to prevent pod and seed infection if significant disease occurs on the foliage earlier in the season.
Despite previous reports of higher than expected levels of ascochyta blight in PBA Samira, experiments using the ascochyta resistant line Samira AR confirmed that resistance in the cultivar is stable. It is presumed that reports of high levels of disease in PBA Samira are due to impure, genetically mixed seed being kept on-farm from past cropping seasons for sowing the following year. This ‘genetic drift’ effect can occur in open pollinated crops when cultivars with different levels of resistance are grown in close proximity. The A. fabae isolates collected from the field to examine the pathogen population dynamics did not cause high levels of disease however growers should always be vigilant in monitoring their crops for signs of disease. Growers should ensure seed kept for future plantings are isolated from other cultivars by a minimum of 200m to minimise the risk of cross-pollination diluting the resistance present in a cultivar.
Table 3. Forty Ascochyta fabae isolates collected in 2017 were inoculated onto a faba bean host differential set in controlled environment conditions in 2018. Entries in the table are the number of isolates per category. ‘AR’ lines are ‘ascochyta resistant’ selections made within the breeding program.
Test reaction | Icarus | Farah AR | PBA Zahra | PBA Rana | Samira AR | Nura |
|---|---|---|---|---|---|---|
R | 1 | 5 | 8 | 15 | 39 | 40 |
MR | 3 | 0 | 12 | 8 | 1 | 0 |
MRMS | 0 | 8 | 18 | 11 | 0 | 0 |
MS | 8 | 23 | 2 | 6 | 0 | 0 |
S | 28 | 4 | 0 | 0 | 0 | 0 |
Note: R = resistant, MR=moderately resistant, MRMS = moderately resistant-moderately susceptible, MS = moderately susceptible; S = susceptible
Resistance to ascochyta blight in chickpeas remains compromised
All chickpea cultivars in the southern region remain rated MS or S to ascochyta blight and require multiple fungicide sprays and a thiram-based seed dressing. Chickpea growers should carefully consider their risk of ascochyta blight infection and ability to effectively control the disease prior to choosing to grow the crop in southern Australia. Despite the dry season in 2018, there were still numerous reports of ascochyta blight in chickpea crops, likely due to the aggressiveness of this pathogen. The breeding program has a number of promising elite lines with improved resistance to ascochyta blight being tested in National Variety Trials (NVT) however these are not due for release until late 2019 and spring 2020.
All chickpea crops will need to be regularly monitored for ascochyta blight infection.
- All chickpea seed must be treated with a thiram-based fungicide prior to sowing to prevent seedling transmission of ascochyta blight onto emerging seedlings.
- Susceptible cultivars will require regular monitoring and may require regular fungicide sprays every two to three weeks throughout the growing season ahead of rain events.
- Moderately susceptible cultivars will generally require three to four strategic sprays ahead of rain events which will offer two to three weeks protection, starting six to eight weeks post sowing.
- Pods of all commercial cultivars are susceptible to ascochyta blight and will require fungicide sprays ahead of rain fronts to protect pods from seed staining and seed abortion.
- Growers must observe a minimum of a three year rotation between chickpeas in the same paddock as the pathogen can survive on stubble and organic matter for several of years. Avoid planting adjacent to last year’s chickpea stubble.
Chickpea fungicide trial demonstrates necessity of fungicide application to control ascochyta blight
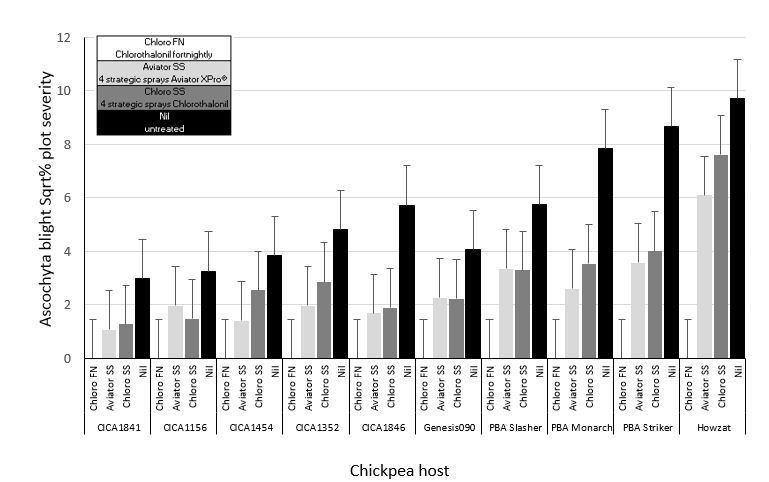
The necessity of regular and timely fungicide application in commercial chickpeas was demonstrated at the Turretfield fungicide trial in 2018. Ten advanced chickpea lines and current Australian commercial cultivars were sown and inoculated with ascochyta blight infested chickpea stubble from the previous season. Plots then received either no spray (nil), fortnightly (FN) chlorothalonil (chloro FN; 1440g ai/ha) starting from 3 to 4 node growth stage and fortnightly thereafter, four sprays of strategically timed (SS) chlorothalonil (chloro SS; 1440g ai/ha) or four sprays of strategically timed Aviator XPRo® (Aviator SS; prothioconazole 90g ai/ha + bixafen 45g ai/ha). The strategic sprays (SS) were applied at early vegetative (3 to 4 node growth stage), pre-canopy closure, early flowering and podding growth stages. All sprays were applied in front of a rainfall event, usually >5 mm of BOM forecasted rainfall.
Fortnightly sprays of chlorothalonil resulted in no disease symptoms in any of the plots (Figure 2). Strategic sprays of either chlorothalonil or Aviator XPRo® significantly reduced disease severity below the nil treatments in all cases except for CICA1156 with Aviator XPRo® and CICA1454 with chlorothalonil. Disease severity in strategically sprayed plots was equivalent between chlorothalonil and Aviator XPro® plots per variety, except for Howzat where Aviator XPro® treated plots had significantly less disease compared to the chlorothalonil treated plots. This may indicate that in extreme epidemics, Aviator XPro® has improved efficacy over chlorothalonil however further testing is required to validate these results.
Figure 2. Ascochyta blight disease severity (square root transformed) per plot in inoculated chickpea trial at Turretfield SA in 2018. Plots were treated with either fortnightly chlorothalonil, 4 strategic sprays of chlorothalonil, 4 strategic sprays of Aviator XPro® or received nil sprays. Vertical bars represent standard errors of the mean. Disease severity range 0-94%. Trial was sown 6 June and rated 4 October in 2018.
Despite the drought conditions, disease was severe enough to cause significant yield losses. Four strategic sprays of AviatorXPro® on Genesis090, PBA Monarch, PBA Striker and the susceptible control Howzat resulted in significant yield gains over the untreated plots. Similarly the four strategic sprays of chlorothalonil resulted in significant yield gains in PBA Monarch and PBA Striker. No significant yield losses occurred in some of the breeding lines, demonstrating effective resistance, albeit in a dry season which limited disease spread.
Susceptible chickpeas demonstrated a good economic return for four sprays of chlorothalonil, even in this dry season. In this low yielding trial, the MS line (Genesis090) only showed a marginal return for spray applications of either chlorothalonil or Aviator XPro®. The higher costs associated with AviatorXPro® make this product only marginally profitable in low yielding seasons, even when disease control is good. The latter fungicide may be better used in medium to high rainfall seasons with higher yielding crops, to ensure an economic return whereas the cheaper chlorothalonil is more economic in the drier lower yielding situations.
Table 4: Grain yield (t/ha) of 10 chickpea varieties under four different fungicide treatment regimes at Turretfield Research Centre, 2018. LSD = 0.171 (p<0.05)
VARIETY | Nil | Aviator SS | chloro FN | chloro SS |
|---|---|---|---|---|
CICA1156 | 0.24 | 0.35 | 0.38 | 0.32 |
CICA1352 | 0.19 | 0.45 | 0.57 | 0.43 |
CICA1454 | 0.33 | 0.42 | 0.4 | 0.35 |
CICA1841 | 0.49 | 0.46 | 0.48 | 0.45 |
CICA1846 | 0.16 | 0.39 | 0.43 | 0.51 |
Genesis090 | 0.24 | 0.42 | 0.42 | 0.35 |
Howzat | 0.02 | 0.25 | 0.52 | 0.16 |
PBA Monarch | 0.04 | 0.35 | 0.5 | 0.33 |
PBA Slasher | 0.23 | 0.38 | 0.56 | 0.38 |
PBA Striker | 0.1 | 0.34 | 0.57 | 0.31 |
Useful resources
Disease management of pulses grown in low to medium rainfall zones: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2018/08/disease-management-of-pulses-grown-in-low-to-medium-rainfall-zones
New pulse variety releases: https://grdc.com.au/resources-and-publications/groundcover/groundcover-137-november-december-2018/new-pulse-variety-releases
Why adhering to integrated ascochyta rabiei management strategy is now more important than ever: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2018/03/why-adhering-to-integrated-ascochyta-rabiei-management-strategy-is-now-more-important-than-ever
Sowing guide for South Australia: https://grdc.com.au/resources-and-publications/all-publications/publications/2018/2018-sowing-guide-for-south-australia
References
Davidson J, Smetham G, Russ M, McMurray L, Rodda, Krysinska-Kaczmarek M, Ford R (2016) Changes in aggressiveness of the Ascochyta lentis population in Southern Australia. Front Plant Sci 7: 393.
Acknowledgements
The research undertaken here is made possible by the significant contributions of growers and agronomists through trial cooperation and provision of diseased plant materials for the isolate collection as well as the support of the GRDC, the author would like to thank them for their continued support. Thank you to Dr Jeff Paull, University of Adelaide for comments on the manuscript and to the technical staff for maintaining trials.
Contact details
Sara Blake
GPO Box 397, Adelaide SA 5001
08 8429 2248
sara.blake@sa.gov.au
@Sara_N_Blake
GRDC Project Code: CUR1403-002BLX, DAS1306-008BLX, DAV1706-003RMX, DPI1607-001RTX,
Was this page helpful?
YOUR FEEDBACK