Can wheat be vernalised during grain development?
Author: Ramon Javier Atayde and Sergio Moroni (School of Agricultural and Wine Science, Charles Sturt University, Wagga Wagga) and Felicity Harris (NSW Department of Primary Industries, Wagga Wagga) and Ben Trevaskis (CSIRO Agriculture and Food, Canberra) | Date: 19 Feb 2019
Take home messages
- Cold temperature during grain development altered phenology in the subsequent generation of vernalisation-sensitive genotypes when seed was retained and grown under controlled conditions.
- The effect of cold temperature during grain development was evident in accelerated early reproductive development and earlier flowering in winter and intermediate spring types.
- Exposure to cold temperature during early vegetative growth was more effective at stimulating development in vernalisation-sensitive genotypes than cold exposure during grain development.
Background
The productivity of wheat is largely influenced by environmental conditions at flowering, with temperature and moisture stress during this period causing significant reductions to yield. The flowering requirements of vernalisation (VRN), photoperiod (Ppd) and earliness per se (EPS) regulate wheat development in response to environmental stimuli, increasing the likelihood of flowering within the optimal window (late September to mid-October in southern NSW). Growers increase the likelihood of coordinating flowering with the optimal window by matching sowing time to variety phenology (Harris et al. 2017).
Literature suggests vernalisation in wheat involves the acceleration of reproductive development in response to cold temperature exposure during early vegetative growth. There is evidence to suggest, however, that cold temperature during grain development may also stimulate a vernalisation response and accelerate development in the subsequent generation (Dobovy et al. 1998; Sharma & Mascia, 1987). The purpose of this honours study was to therefore investigate the effects of cold temperature during grain development on flowering time in the subsequent generation.
Method
Eight near isogenic lines (NILS) derived from cv. Sunstate (developed by Ben Trevaskis, CSIRO) and three winter varieties (Marombi, EGA Wedgetail and Wylah) with a variety of sensitivities to photoperiod and vernalisation were used in the study (Table 1) (Eagles et al. 2010; Trevaskis, 2010). This paper presents results from the wheat NILS W7, W29 and W77 (cv. Sunstate), encompassing treatment responses from winter, intermediate spring and fast spring genotypes.
Table 1.Genotype, flowering requirements (vernalisation and photoperiod) and associated phenology type of wheat used in the study.
Genotype | Photoperiod response | Vernalisation response | Phenology type |
|---|---|---|---|
W34 | None | None | Fast spring |
W77 | None | None | Fast spring |
W40 | Weak | None | Spring |
W29 | None | Weak | Intermediate spring |
W71 | None | Weak | Intermediate spring |
W7 | None | Strong | Winter |
W8 | None | Strong | Winter |
W46 | Moderate | Strong | Slow winter |
EGA Wedgetail | Weak | Strong | Winter |
Wylah | None | Strong | Winter |
Marombi | Weak | Very strong | Slow winter |
Seed from of all eleven genotypes was sown and initially grown for six weeks at 4°C under short day conditions (8-hour light period @ 200μmols/m2) to saturate any vernalisation requirements and synchronise development. Seedlings were then transferred into a growth chamber (Conviron PGW40) set to a constant 23°C under 16-hour light periods @ 800μmols/m2, where they remained until the detection of flowering. Fourteen days after flowering was observed, mother plants and developing grains were exposed to cold treatments.
Treatments consisted of exposure to constant cold temperature (4°C) for 0, 21, 28, 35 and 42 calendar days. Post-treatment, mother plants and developing grains were transferred to a chamber set to 23°C day/15°C night temperatures under 16-hour light periods @ 800μmols/m2 and allowed to mature prior to harvest.
To determine treatment effects on development in the subsequent generation, harvested seed was sown into a temperature-controlled glasshouse (23°C day/18°C night temperatures ±3°C) under long days (16-hour light periods) to saturate photoperiod requirements. A fully-vernalised (FV) treatment was also included to determine whether cold temperature during grain development had a similar effect on phasic development as cold temperature during early vegetative growth.
The FV treatment involved exposing untreated (T0) seed to cold treatment (4°C under short days/8-hour light periods @ 200μmols/m2) from sowing. FV plants remained under cold treatment for 42 days to fully saturate vernalisation requirements in the winter genotypes used.
Phenological measurements were recorded every second day ±1 between sowing and flowering (Z65), with growth stage scored using the Zadoks scale (Zadoks et al. 1974). A subset of plants was also sampled at Z14 to observe treatment effects on early reproductive development. Plants were dissected and apical meristems observed under a microscope before being allocated a score using a modified scale from Bonnett (1966).
Effects on early apical development
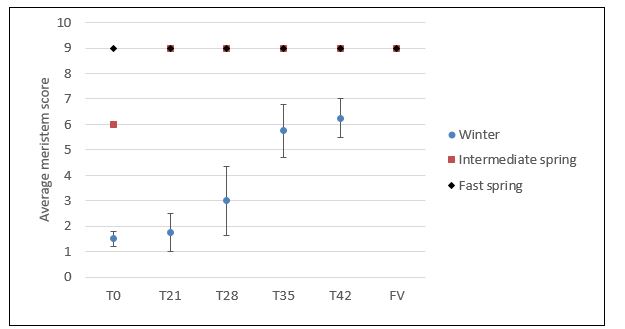
Plants of intermediate spring types (weak vernalisation requirement) and winter types (strong vernalisation requirement) grown from cold-treated seed were significantly more advanced than untreated plants. Differences in apical development between treatments suggested a dose response, whereby longer periods of cold treatment (>21 days) resulted in faster apical development (higher meristem score) (Figure 2).
The winter type (W7) had the greatest response to cold treatment, which is understandable given its obligate vernalisation requirement. The intermediate spring type (W29) also responded to cold treatment due to its facultative vernalisation requirement. Progression to early reproductive development was not dependent on cold treatment as apices from the T0 treatment were also reproductive (meristem score 6 – indicative of spikelet forming branches). At the time of sampling, apices from all treatments of the fast spring type (W77) had progressed to terminal spikelet (Z30), reflective of its insensitivity to vernalisation.
It was also noted that the apices of plants exposed to cold temperatures during early vegetative growth (FV) were more reproductive (meristem score 9 – terminal spikelet) than apices from the T42 treatment, despite similar periods of cold exposure. This suggests that cold temperature during early vegetative development has a greater impact on apical developmental rate.
Figure 2.The effect of cold temperature during grain development on the timing of reproductive development in apical meristems of winter, intermediate spring and fast spring wheats. Wheat apical meristem scoring system adapted from Bonnett et al. (1966). 1. Vegetative shoot apex with leaf primordia; 2. Vegetative shoot apex with leaf primordia; 3. Elongated shoot apex; 4. Beginning of spikelet formation shown by double ridges; 5. Early stages of spikelet formation; 6. Spikelet-forming branches just before differentiation of spikelet parts; 7. Beginning of differentiation of the empty glumes; 8. Basal florets initiated in the middle spike; 9. Florets of all the spikelets have been initiated.
Effects on flowering time
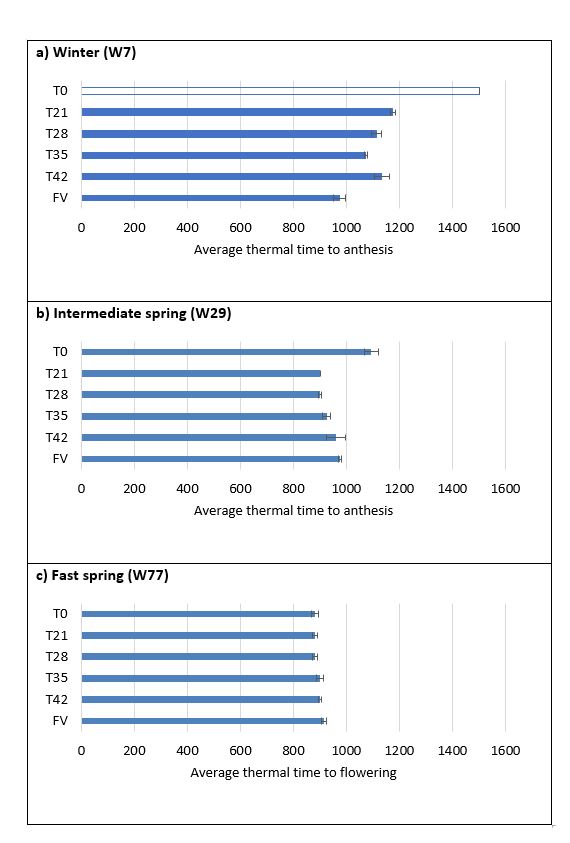
Differences in the average thermal time to flowering were consistent with observations on early apical development, whereby cold treated plants flowered earlier than untreated plants in both the winter and intermediate spring types. Again, the fast spring type flowered at a similar time regardless of treatment (Figure 3). Results also indicated a dose response, whereby progressively longer periods of cold temperature exposure (>21 days) resulted in faster flowering times (Figure 4). Faster flowering in the FV plants compared to the T42 treatment agrees with the suggestion that cold temperature during early vegetative growth has a greater effect on phasic development than cold temperature during grain development.
Figure 3.The effect of cold temperature during grain development on average thermal time to flowering for a) winter, b) intermediate spring, and c) fast spring wheats.
In southern NSW, the chances of experiencing 21 to 42 days of cold temperatures (4°C) during grain development are unlikely. However, it is not uncommon for temperatures to fall within the vernalising range (0°C - 15°C) during late September to early October for short periods of time (<21 days). It is, therefore, feasible that the observations from this experiment will have relevance in the field, particularly for intermediate spring types with a weak vernalisation requirement.
Conclusion
The results of this study have shown that the effects of cold temperature during grain development can persist in the developing and mature grain and have an effect on phasic development in the subsequent generation. Specifically, it is possible to saturate the vernalisation requirements of intermediate spring and winter types through prolonged exposure to cold temperature during grain development. This results in accelerated reproductive development at the apical meristem and an earlier flowering time. Results also suggest that a) the effect of cold temperature treatment is dose responsive, whereby prolonged exposure to cold temperatures resulted in more rapid development, and b) that vernalisation responses are saturated more effectively during early vegetative development than during grain development. While this study has been conducted under controlled environments, future research should focus on the implications of this phenomenon under field conditions in southern NSW.
References
Bonnett, O. T. (1966). Inflorescences of maize, wheat, rye, barley, and oats: their initiation and development. Bulletin (University of Illinois (Urbana-Champaign campus). Agricultural Experiment Station).
Dobovy, V. I., Hamula, P. V., & & Khamula, P. (1998). Vernalisation of winter wheat seeds in in vitro culture of a growing spike and seed embryos. National Academy of Sciences of Ukraine, 30(5), 391-396.
Harris, F. A., Eagles, H. A., Virgona, J. M., Martin, P. J., Condon, J. R., & Angus, J. F. (2017). Effect of VRN1and PPD1 genes on anthesis date and wheat growth. Crop and Pasture Science, 68(3), 195-201. doi:https://doi.org/10.1071/CP16420
Eagles, H. A., Cane, K., Kuchel, H., Hollamby, G. J., Vallance, N., Eastwood, R., Martin, P. J. (2010). Photoperiod and vernalisation gene effects in southern Australian wheat. Crop and Pasture Science, 61(9), 721-730.
Sharma, H. C., & Mascia, P. N. (1987). Vernalisation of immature embryos of winter wheat genotypes. Euphytica, 36(1), 161-165. doi:10.1007/bf00730659
Trevaskis, B. (2010). The central role of the VERNALISATION1 gene in the vernalisation response of cereals. Functional Plant Biology, 37(6), 479-487. doi:https://doi.org/10.1071/FP10056
Zadoks, J. C., Chang, T. T., & Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed research, 14(6), 415-421.
Acknowledgements
The research undertaken in this honours project is made possible by the significant financial contributions of the EH Graham Centre, NSW Department of Primary Industries and CSIRO.
Contact details
Ramon Javier Atayde
0449 933 309
javi.atayde@gmail.com
Was this page helpful?
YOUR FEEDBACK