Crown rot – what is the threat coming out of a dry year?
Author: Steven Simpfendorfer (NSW DPI, Tamworth) and Alan McKay (SARDI, Adelaide) | Date: 19 Feb 2019
Take home messages
- Dry seasons which reduce crop biomass production and yield still increase crown rot inoculum levels within paddocks, but to a lesser extent than in good seasons.
- Decomposition of previous cereal stubbles which harbour the crown rot pathogen, even under break crops (e.g. canola), is reduced in dry seasons. Hence, inoculum levels may not have declined significantly since harvest of 2017 cereal crops.
- PREDICTA® B is a reliable technique for assessing the risk of crown rot and a range of other soil-borne or stubble-borne pathogens prior to sowing in 2019.
- PREDICTA® B also has new tests for sclerotinia stem rot in canola as well as pulses and yellow spot in wheat. Tests for septoria tritici blotch of wheat and net blotches in barley are under consideration for next season.
- Follow sampling recommendations in the manual V10.2, including the addition of pieces of cereal stubble to improve the detection of stubble-borne pathogens such as the crown rot fungus.
Background
Crown rot, caused predominantly by the fungus Fusarium pseudograminearum, is a significant disease of winter cereals across Australia, including southern NSW. Infection is characterised by a light honey-brown to dark brown discolouration of the base of infected tillers, while major yield loss from the production of whiteheads is related to moisture and/or temperature stress post-flowering. It is critical to understand that there are three distinct and separate phases of crown rot, namely survival, infectionand expression. Management strategies can differentially affect each phase.
Survival
The crown rot fungus survives as mycelium (cottony growth) inside winter cereal (wheat, barley, durum, triticale and oats) and grass weed residues, which it has infected. The crown rot fungus will survive as inoculum inside the stubble for as long as it remains intact, which varies greatly with soil and weather conditions as decomposition is a very slow process. The crown rot fungus has been shown to survive as mycelium for up to three years in infected cereal residues (Summerell and Burgess 1988).
Infection
Given some level of soil moisture, the crown rot fungus grows out of stubble residues and infects new winter cereal plants through the coleoptile, sub-crown internode or crown tissue which are all below the soil surface. The fungus can also infect plants above ground right at the soil surface through the outer leaf sheathes. However, with all points of infection, direct contact with the previously infected residues is required and infections can occur throughout the whole season given moisture. Hence, wet seasons favour increased infection events by the crown rot fungus and when combined with the production of greater stubble loads significantly builds up inoculum levels.
Expression
Yield loss is related to moisture/temperature stress around flowering and through grain-fill. This stress is believed to trigger the crown rot fungus to proliferate in the base of infected tillers, restricting water movement from the roots through the stems, and producing whiteheads that contain either no grain or lightweight shrivelled grain. The expression of whiteheads in plants infected with crown rot (i.e. still have basal browning) is restricted in wet seasons and increases greatly with increasing moisture/temperature stress during grain-fill. Focus attention to crops around trees within a paddock or along tree lines. Even in good years, whiteheads associated with crown rot infection are likely to be seen around trees. This is due to the extra competition for water.
Correct diagnosis is important
In southern NSW in 2018, the expression of whiteheads from crown rot was confused with stem frost and/or drought tipping of wheat heads. In wetter seasons, whiteheads associated with crown rot can also be confused with those caused by a different fungal pathogen (Gaeumannomyces graminis var. tritici) which causes take-all (hay-die). Whiteheads (deadhead) caused by crown rot can be confirmed by tracking that head down to the tiller base which will always have a brown discolouration under the leaf sheath. Deadheads associated solely with stem frost will not have brown tiller bases but tend to have blistering on the stem at the point where the frost formed. This is quite often at a uniform height in adjacent plants. Drought stress tends to make the tip of the heads die rather than the whole head and equally will not have brown tiller bases if crown rot is not also present.
Take-all and crown rot can also be differentiated easily. Take-all causes all heads on an infected plant to form whiteheads while individual tillers normally only produce whiteheads with crown rot, unless the severity of infection is very high. Take-all causes the tiller bases to have a black rather than honey brown appearance, as with crown rot. Take-all infected plants will also have areas of blackened roots. Take-all affected plants are easily pulled out of the ground, while plants affected by crown rot are generally anchored well. However, plants with severe crown rot may snap at the lowest nodes if plants are pulled from dry soil around harvest. Crown rot can infect roots, but it does not cause the same level of damage as take-all.
If you are unsure, then samples can be submitted to NSW DPI pathology services at Wagga Wagga or Tamworth for confirmation.
But we don’t get crown rot in southern NSW
Even with recent research on the distribution and management of crown rot in southern NSW farming systems (Milgate and Baxter 2018), some growers, advisers and researchers are still surprised when whiteheads appear in wheat crops in this region, such as in 2018. The crown rot fungus is a stubble-borne pathogen which hosts inside residue of all winter cereals and grass weeds (especially barley grass, phalaris, annual ryegrass and wild oats). Grass weeds are a major concern as they not only act as a source of crown rot inoculum, but also compete for soil water during the crop or fallow phase, which can induce crop stress and exacerbate the expression of whitehead. The increased adoption of stubble retention practices has seen an associated rise in the incidence of fusarium crown rot globally. So why would southern NSW be any different?
So, to answer the following statements:
- We don’t get crown rot in southern NSW. Yes, you do. Medium to high levels of infection occur in some paddocks. Southern NSW tends to have less stress during grain-filling which reduces the expression of whiteheads, except in dry seasons such as 2018.
- Crown rot is only a disease of dry seasons. Incorrect. Crown rot infection is favoured by moist conditions and increased cereal biomass in wetter seasons increases potential inoculum loads (Table 1). Rather, the expression of whiteheads in tillers infected with crown rot only occurs when dry and/or hot conditions occur during grain filling.
- Cropping systems in southern NSW are favouring build-up of crown rot inoculum within paddocks with infection of cereal crops occurring in all seasons. We simply tend to get lower expression of whiteheads associated with crown rot infection, except in seasons with dry/hot conditions during grain filling. Correct.
A quantitative DNA-based soil testing service, PREDICTA® B, is available in Australia to assist grain growers to predict the likely risk of soil-borne and stubble-borne diseases by measuring pathogen levels prior to planting. Growers have the option of changing varieties or modifying cropping programs in situations where the risk of crop loss is high. Presenting PREDICTA® B results mapped to each town as pie charts helps to highlight higher risk cropping areas for different diseases, such as crown rot, prior to sowing which can be used to inform industry. Note, the size of each pie chart is proportional to the number of samples mapped to the town and the numbers of low, medium and high disease risk samples are presented as green, orange and red sectors, respectively (Figure 1, n.b. colour version available)
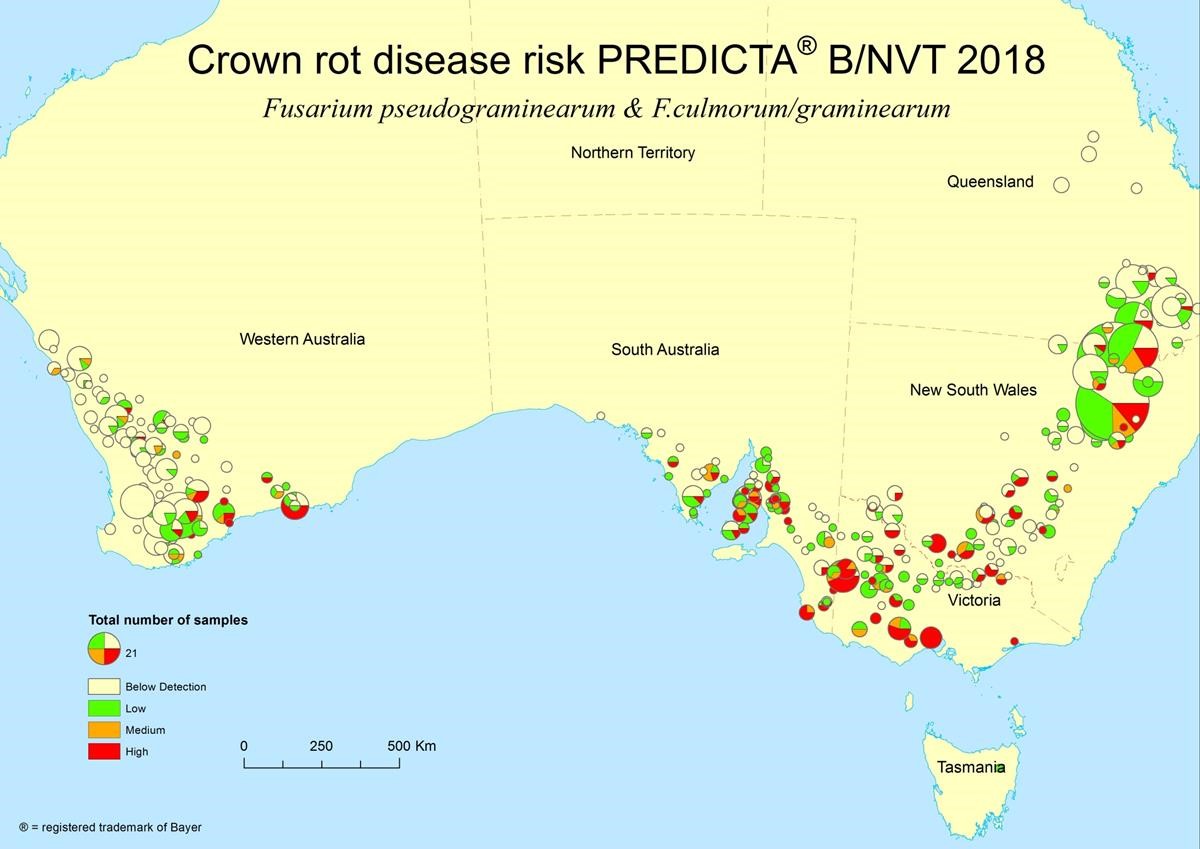
Figure 1. Distribution and levels of Fusarium spp. associated with causing crown rot prior to sowing in 2018 (Source: PIRSA).
Although the level of PREDICTA® B testing (size of each pie chart and number) is generally lower in southern NSW, especially in central NSW, than other regions of Australia, there were still a considerable number of the paddocks tested with a medium (orange) to high (red) risk of developing crown rot in 2018 (Figure 1). Encouragingly, there are a higher proportion of paddocks in southern NSW where inoculum of the crown rot fungi were not detected (white colour). The build-up of inoculum levels is very much on a paddock-by-paddock basis dependent on initial presence of the pathogen, rotation (frequency of cereals), grass weed control, stubble management, row placement and biomass production (yield).
Inoculum of the crown rot fungus is present in many paddocks in southern NSW (Figure 1). However, the increased reliability of rainfall plus cooler relative temperatures during flowering and grain-filling, especially in south-east NSW, normally limits expression of whiteheads in crown rot infected tillers.
What happens to crown rot inoculum in a dry year?
Just remember the key fact here is that the crown rot fungus is a stubble-borne pathogen of cereal crops and grass weeds. Hence, inoculum levels are a function of the number of plants infected with the crown rot fungus and the biomass (number of tillers) produced by the cereal crop. A simple equation was developed by Backhouse (2006) to forecast likely crown rot infection levels in successive wheat crops where:
Incidence of infection in 2019 = 5.25x square root of (incidence of infection in 2018 x yield)
In the equation, 5.25 is an average infection constant developed from field experiments and yield of the previous wheat crop is taken as a surrogate for biomass (Backhouse 2006). The equation can be used to highlight the likely impact of a dry season, with reduced biomass production (crop yield), on crown rot inoculum levels and hence infection in a following cereal crop (Table 1).
A similar level of crown rot infection in 2019 (23%) will occur in theory following a failed crop (1.0t/ha) with 20% infection in 2018, or a moderate yielding crop (2.0t/ha) with 10% infection in 2018, or even a good crop (4.0t/ha) with 5% infection in 2018 (Table 1). Note that the incidence of infection in 2018 is not based on expression of whiteheads as this can underestimate crown rot levels. It should be based on the incidence of characteristic basal browning or plating to recover the causal pathogen Fp. This process is simplified by using PREDICTA®B testing prior to sowing.
Table 1. Simple model predicting the incidence of crown rot infection in cereal crops in 2019 (Backhouse 2006).
Cereal yield in 2018 (t/ha) | |||||
|---|---|---|---|---|---|
% infection 2018 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 |
| 5 | 12 | 17 | 20 | 23 | 26 |
| 10 | 17 | 23 | 29 | 33 | 37 |
| 20 | 23 | 33 | 41 | 47 | 53 |
| 30 | 29 | 41 | 50 | 58 | 64 |
| 40 | 33 | 47 | 58 | 66 | 74 |
| 50 | 37 | 53 | 64 | 74 | 83 |
Decomposition of previous cereal stubbles which harbour the crown rot pathogen, even under break crops (e.g. canola), is reduced in dry seasons. Inoculum levels may not have declined significantly since harvest of 2017 cereal crops. Hence, dates in Table 1 could be changed to reflect cereal crops grown in 2017 in regards to measured crown rot infection levels and yield, even though a canola break crop may have been grown in 2018.
The actual risk of yield loss from crown rot in 2019, however, is more complicated as it is a function of inoculum levels present at sowing (PREDICTA®B or other assessment), cereal crop and variety grown, soil water storage, sowing time, sowing implement, row placement, biomass production (e.g. nitrogen (N) nutrition and tiller number/m2), in-crop rainfall and temperature during grain filling (i.e. evapotranspiration stress). Unfortunately, an equation does not currently exist to determine the actual risk of yield loss from crown rot.
Crown rot detection using PREDICTA®B
PREDICTA®B is a reliable technique for assessing the risk of crown rot and a range of other soil-borne or stubble-borne pathogens prior to sowing in 2019. PREDICTA®B has been used extensively in recent crown rot experimentation in southern NSW (Milgate and Baxter 2018).
Additionally, studies undertaken in collaboration with National Variety Trial (NVT) and National Paddock Survey (NPS; BWD00025) projects, showed that avoiding stubble when collecting PREDICTA®B samples led to a significant under estimation (failure to warn) of the risk of crown rot. This can be fixed by adding one 5cm piece of stubble (including crown) from the base of the previous winter cereal or grass weed plants (one to several years old) from the 15 different locations where soil cores are collected. Adding stubble in this way also facilitates testing for other stubble-borne pathogens (e.g. yellow leaf spot and septoria tritici blotch in wheat or net blotches in barley, when tests for the latter are added).
PREDICTA® B new developments
PREDICTA® B is under continual development to provide a comprehensive assessment of the levels of soil-borne and stubble-borne pathogens that pose a potential risk to cereals and increasingly, pulse and oilseed crops. The aim is to provide a fast and cost-effective way for growers to determine the soil-borne and stubble-borne disease risks within a paddock, to help inform decisions of crop and variety choice and guide management to minimise losses.
Further information on the ‘tests under evaluation’ can be found here. SARDI accredited consultants can obtain information on new tests and management options in Version 10 of the Broadacre Soilborne Disease Manual, available here.
To fast track delivery of new tests, these results are reported with categories based on population density so growers and consultants can benchmark levels of pathogen DNA detected in paddocks against the rest of industry. When the relationship between the initial pathogen level and disease has been defined, the level detected in the sample is reported with a disease risk rating. Of particular interest to southern NSW cropping systems is a new test reporting population densities of Sclerotinia sclerotiorum, which causes sclerotinia stem rot in canola and pulse crops (Figure 2 n.b. colour version available here). A further test for population densities of Pyrenophora tritici-repentis, the cause of yellow spot in wheat, is also being reported in 2019. Tests for septoria tritici blotch of wheat and net blotches in barley are also currently being implemented.
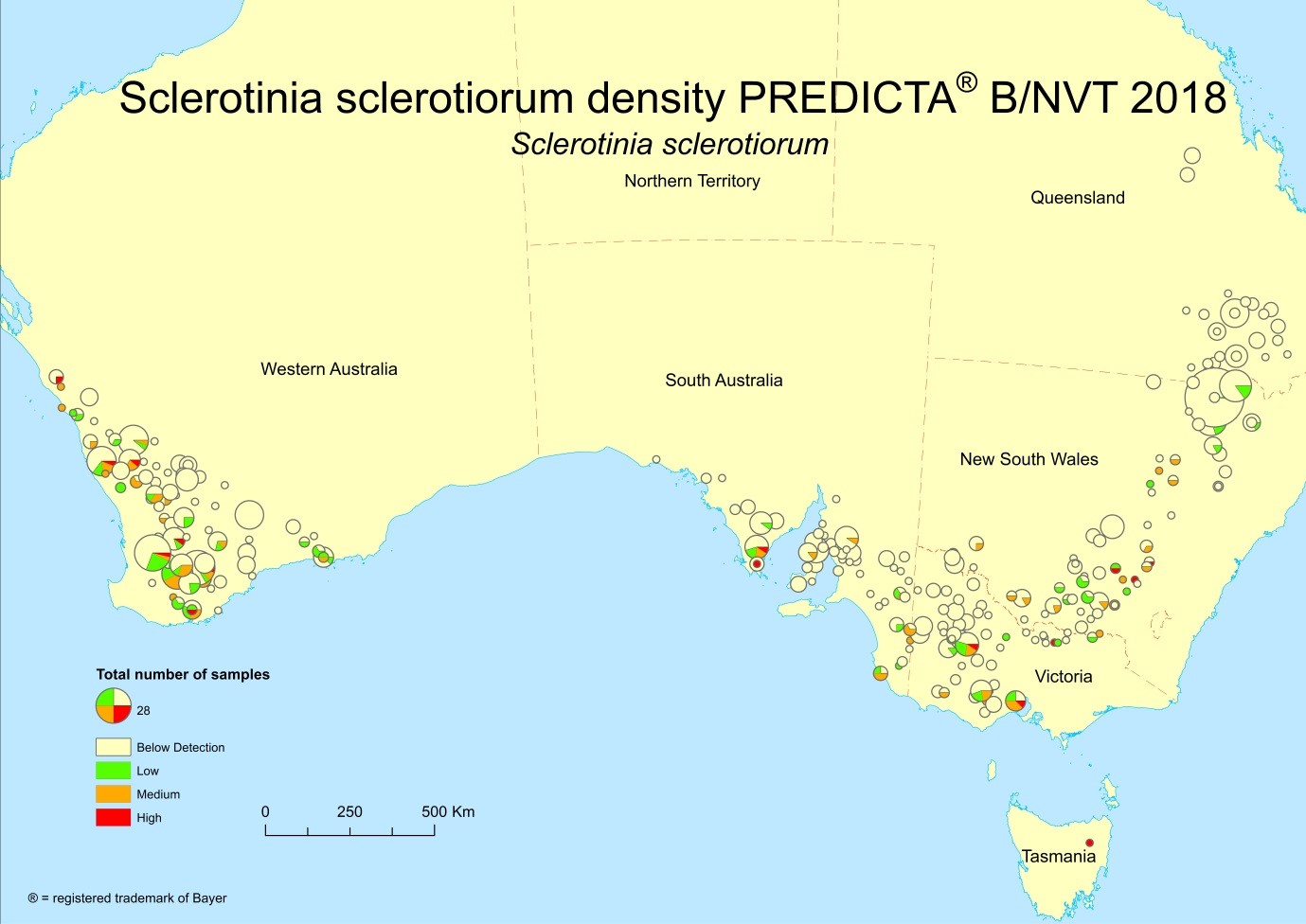
Figure 2. Distribution and levels of Sclerotinia sclerotiorum prior to sowing in 2018.
Conclusions
Southern NSW is not immune from crown rot in cereal crops. Dry seasonal conditions (e.g. 2018) favour the expression whiteheads, making the disease more conspicuous to growers, advisers and even researchers. Longer term management to reduce losses from crown rot requires understanding of the pathogen across seasons whether they be wet, dry or in between. PREDICTA®B offers a reliable technique to monitor crown rot inoculum levels across seasons (wet and dry); rotation sequences; and the success of varying integrated management strategies which may be implemented, but do not forget to add stubble to the soil sample. A single PREDICTA®B test further allows levels of a range of other soil-borne and stubble-borne pathogens (e.g. rhizoctonia root rot, take-all, root lesion nematodes) to be determined with newer tests for yellow spot in wheat, along with sclerotinia stem rot in canola and pulses recently being included.
Useful resources
Correct Sampling 'A Must' To Accurately Expose Disease Risk
References
Backhouse D (2006). Forecasting the risk of crown rot between successive wheat crops. Aust J Exp Agric 46, 1499-1506.
Milgate A, Baxter B (2018). Management of crown rot in southern NSW farming systems. GRDC Update. https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2018/02/management-of-crown-rot-in-southern-nsw-farming-systems
Summerell BA, Burgess LW (1988). Stubble management practices and the survival of Fusarium graminearum Group 1 in wheat stubble residues. Aust Plant Path 17, 88-93.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the author would like to thank them for their continued support.
Contact details
Steven Simpfendorfer
NSW DPI
4 Marsden Park Rd, Tamworth NSW 2340
0439581672
steven.simpfendorfer@dpi.nsw.gov.au
@s_simpfendorfer
Alan McKay
SARDI
Adelaide SA 5064
08 8429 2216
alan.mckay@sa.gov.au
PREDICTA® is a registered trademark of Bayer.
Was this page helpful?
YOUR FEEDBACK
