Effects of natural enemies on green peach aphids in Western Australia
Author: Dusty Severtson, DPIRD Northam, Alan Lord, DPIRD South Perth, Sarina Macfadyen, CSIRO Black Mountain | Date: 26 Feb 2019
Key Messages
- Lacewing larvae kept green peach aphid (GPA) populations on caged canola plants the same or lower while the GPA populations in control cages increased substantially.
- Spotted ladybird beetles decreased GPA populations slightly during the first week but then GPA populations increased markedly but were still lower than controls.
- Parasitoid wasps were more effective at limiting or decreasing GPA populations when they initially encountered lower GPA populations (i.e. higher wasp to aphid ratio) than when they encountered high GPA populations (i.e. low wasp to aphid ratio).
- Yellow sticky traps may be an easy way of monitoring parasitoid wasps in the field which could be related to aphid populations and potential for biological control.
Background
Research in many agricultural industries has shown that commonly observed natural enemies of aphids, including predators (e.g. ladybird beetles, lacewing larvae) and parasitoid wasps, have the potential to limit green peach aphid (Myzus persicae; GPA) population growth or even eliminate local populations. Commonly used broad-spectrum insecticides kill these natural enemies which may otherwise be useful in limiting aphid populations to below damaging levels. A greater understanding of the scenarios and factors which drive biological control of GPA by natural enemies in WA field crops would provide further incentive to use insecticides judiciously and, where possible, encourage use of selective insecticides which target pests and conserve the natural enemies.
Larger invertebrate predators, such as ladybird beetles, lacewings and hoverfly larvae are often detected and counted in sweep nets when monitoring canola crops during winter and spring. However, adult parasitoid wasps are difficult to identify in sweep net samples amongst plant debris etc. and difficult to count given their small size. Their activity in a field can be inferred from the presence of mummified aphids. These brown/bronze coloured mummies or aphid shells are the result of a parasitoid wasp inserting an egg into a live aphid which hatches into a larva and feeds on the aphid ultimately killing it and emerging from the mummy as an adult wasp. Flying insects including aphids and natural enemies are routinely trapped and counted using yellow sticky traps, which are commonly used to monitor aphids and other flying insects in agriculture, especially horticulture (see Fig. 1). These may be useful in monitoring parasitoid wasps in field crops which could be associated with aphid populations.

Figure 1. Yellow sticky trap used to collect flying insects such as aphids and parasitoid wasps.
Aims
The aim was to experimentally assess the effects of common natural enemies on green peach aphid populations in field cages and openly infested (i.e. ‘sentinel) plants, and to demonstrate the use of yellow sticky traps in monitoring resident parasitoid wasp populations.
Method
Two field trials were conducted during 2017-18.
Trial 1: Ladybird beetles and lacewing larvae
The first experiment was conducted at the Great Southern Agricultural Research Institute, Katanning, WA in 2017 and consisted of one hectare of canola cv. Bonito, seed-treated with Gaucho® (imidacloprid) and Jockey Stayer® (fluquinconazole) at label rates. The canola seed was sown into oat stubble on 16 May at 5 kg/ha. A post-sowing pre-emergent application of Pyrinex Super® (chlorpyrifos and bifenthrin) was applied at 1 L/ha. No further insecticide applications were applied during the season. Field cages produced by SARDI consisted of a plastic pipe infrastructure with a circular plastic base partially buried in the ground, stabilised by iron rods driven into the soil and enclosed with a hook-and-loop fastened insect-proof mesh with a zip at the top for plant/insect assessments. Cages were erected over single plants with 4 treatments replicated 6 times in a randomised block design. On 28 August, all plants were infested with approx. 100 GPA. On 4 September, GPA were counted on all plants to ensure aphid establishment and consistency in numbers. Cages within blocks were distanced approx. 3 m from each other and blocks approx. 20 m apart. Treatments were: 1) controls (closed cages with 100 GPA and no predators), 2) sentinels (open cages with 100 GPA, no predators, and cage mesh removed to encourage visitation by resident natural enemies, 3) closed cages with 100 GPA and two spotted ladybird beetles (Harmonia octomaculata) and 4) closed cages with 100 GPA and five second instar green lacewing (Mallada signata) larvae. The predators were purchased from Bugs for Bugs . Winged and wingless GPA were counted on all plants at 0, 3, 7 and 14 days after treatment. Predators were not counted on assessment days as they were often hiding at the time of plant inspections.
Trial 2: Parasitoid wasps
The second experiment was conducted in 2018 at Northam, WA consisting of one hectare of canola cv. Hyola 559TT treated with Cruiser Opti® (thiamethoxam, lambda cyhalothrin) and Jockey Stayer® (fluquinconazole) by the manufacturer. A post sowing pre-emergent application of Talstar® (bifenthrin) was applied at 100 mL/ha. No further insecticide applications were applied during the season. The field cages were installed as described above but with 3 plants per cage, and 8 treatments were allocated in 4 replicates. The 4 caged GPA treatments were 20, 100, 200 and 500 GPA per plant each with 3 freshly emerged (from pupae) and mated female wasps (Diaretiella rapae) which were reared at SARDI, SA. The 100 and 200 GPA treatments had controls (i.e. no parasitoid wasps) for comparison. Further to these 6 plant cages per block, 2 plants were chosen at random near each block and flagged as sentinel plants; these were infested with 100 and 200 GPA but without cages. Winged, wingless and mummified (i.e. wasp-parasitised) aphids were counted on each plant at 0, 14, 28 and 42 days from wasp introduction up to a maximum count of 10 000.
Yellow sticky traps
The one-hectare of canola at Northam, WA was monitored for aphids and natural enemies using yellow sticky traps. Five double-sided yellow sticky traps were erected on star pickets (at crop height) and replaced fortnightly from 17 July to 17 October, 2018. Four were positioned at the middle of each crop side (N, S, E and W) 10 m inwards from crop edge, with the fifth positioned in the middle. Sticky trap sides faced an east/west direction.
Results
Ladybird beetles and lacewing larvae
GPA within control cages increased consistently over the 14 day period (Fig. 2). Lacewing larvae kept GPA populations the same or lower indicating significant biological control compared to control cages, especially at 14 days after treatment (DAT). Spotted ladybird beetles decreased GPA populations slightly during the first week compared to control cages, but then GPA increased on most plants (although numbers after 14 days were still significantly lower than the control). GPA decreased on the sentinel plants (i.e. open cage) after 14 days, indicating that either resident predators and/or environmental factors (e.g. rainfall, wind) impacted the GPA on these plants. Winged aphids were not included in Fig. 2 as no plant averaged above 1 winged GPA per plant.
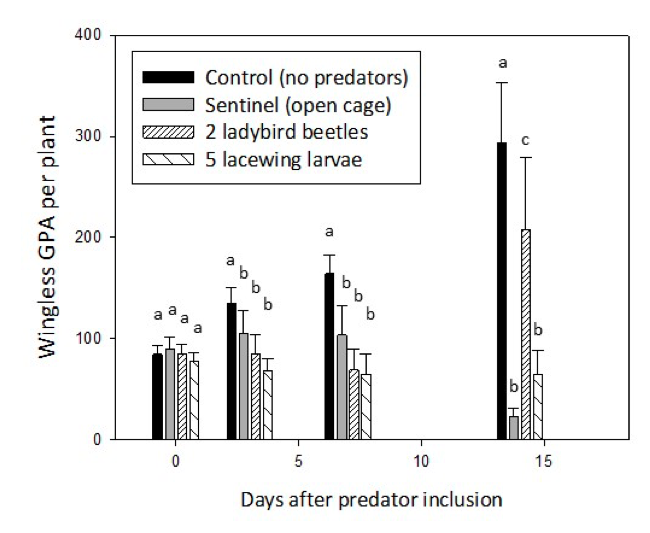
Figure 2. Bar charts of wingless green peach aphid numbers per plant (N=6) for the treatments: control - closed cage with no predators, sentinel - open cage with no predators added, closed cage with 2 spotted ladybirds and closed cage with 5 green lacewing larvae at 0, 3, 7 and 14 days after predator introduction. Error bars indicate standard error of the mean. Like letters indicate like means at P<0.05.
Parasitoid wasps
The effects of adding three female parasitoid wasps to cages on the populations of wingless, winged and mummified GPA are displayed in Figure 3. GPA generally increased in number exponentially in the controls (Figs 3e, 3f; note the logarithmic scale). GPA in the 100 GPA treatment (Fig. 3b) increased to levels similar to the control, despite an average of 34 and 103 mummies being detected 28 and 42 days after treatment (DAT), respectively. GPA in the 200 GPA treatment increased to levels similar to the control up until 42 DAT, which showed averages of 6345 and over 10 000 GPA, respectively. These differences suggest that the parasitoid wasps alone were not providing much aphid control in these treatments. The highest wasp to GPA ratio (1:7) was in the 20 GPA treatment. Here, average GPA increased to only 108 wingless and 16 winged aphids by 42 DAT with 25 mummies counted. Alternatively, the 500 GPA treatment resulted in the most mummies which was an average of 442 at 42 DAT, with one plant holding 1450 mummies. Wingless GPA numbers in the 500 GPA treatment averaged 520, 1242, 8625 and over 10 000 at 0, 14, 28 and 42 DAT. There were also over 10 000 winged aphids indicating high levels of overcrowding of GPA within plant cages. The opposite pattern was seen on the sentinel plants, with a reduction in GPA numbers per plant over the trial period. It is not known which natural enemies and/or environmental conditions were responsible for the significant reductions in GPA. Mummies counted on these plants indicate partial attribution to resident parasitoid wasps which have mostly been identified as Diaretiella rapae (see sticky trap details below).
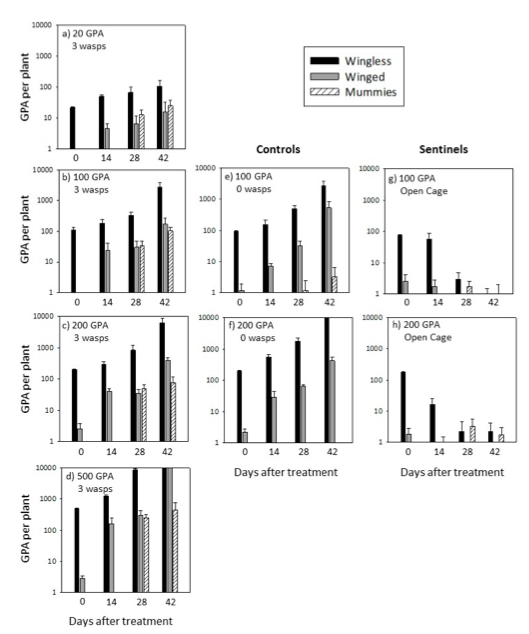
Figure 3. Results of wingless, winged and mummified green peach aphids (GPA) at initial canola plant infestations of 20 (a), 100 (b), 200 (c) and 500 (d) GPA at 0, 14, 28 and 42 days after 3 newly mated female parasitoid wasps (Diaretialla rapae) were added to plant cages. Control plants (i.e. no wasps added) are displayed at e and f, and sentinel plants (i.e. cage tops removed to attract resident natural enemies to GPA-infested plants) are displayed at g and h. Note that the y-axis is a logarithmic scale. Error bars indicate standard error of the mean.
Although the three female parasitoid wasps in each plant cage were not able to parasitise and reproduce fast enough to overcome GPA reproduction rates and reduce GPA numbers in the various treatments, the study did show that the wasps produced more mummies when exposed to higher populations of GPA (see Fig. 4a). Higher ratios of mummies to GPA were evident as the wasps encountered lower numbers of GPA, especially at 28 DAT (see Fig. 4b). These ratios decreased after 28 DAT as the GPA increased in number much more rapidly than mummies. Therefore, the data indicates that the parasitoid wasps are more effective in reducing GPA numbers in scenarios which favour higher wasp or mummy to GPA ratios. High wasp or mummy to GPA ratios may be achieved by either relatively low numbers of wasps visiting canola plants with low numbers of GPA, or due to high numbers of wasps visiting low, medium or even high numbers of GPA.
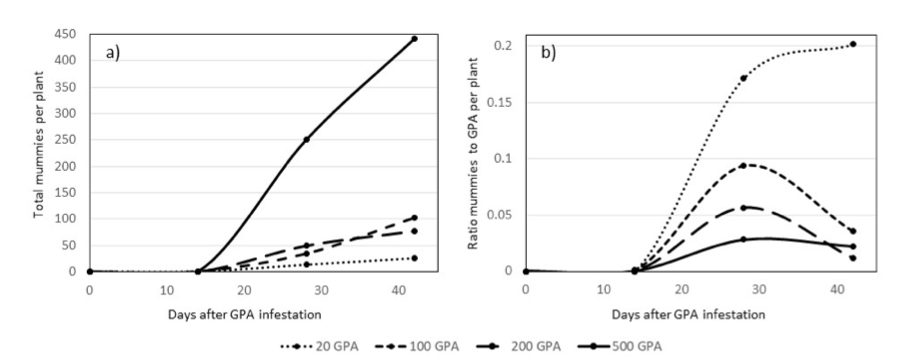
Figure 4. Means of total GPA mummies per plant (a) and ratio of mummies to total GPA per plant (b) at 0, 14, 28 and 42 days after introduction of 3 female parasitoid wasps (Diaretiella rapae) into plant cages containing 20, 100, 200 and 500 GPA.
Monitoring parasitoid wasps using sticky traps
The sticky traps were efficient in trapping parasitoid wasp and winged aphid populations in the canola crop. Results showed a clear trend of increasing parasitoid numbers during July-October, 2018 (Fig. 5). Although not consistently monitored, canola plants were inspected for pests during this time, and green peach and cabbage aphids were occasionally detected on plants, especially along the crop edges. It was noted that these populations disappeared instead of increase, and validation of this being related to natural enemies would require experimental assessment. The final sticky traps were collected on 17 October, and it was noted that clusters of cabbage aphids could be seen on approx. 5% of plants along the crop edges. This immigration and increase of cabbage aphids was consistent with the winged aphids counted on the sticky traps.
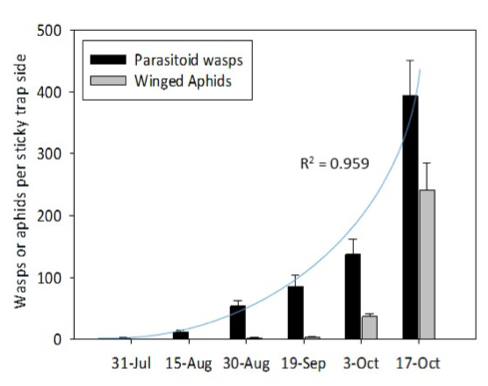
Figure 5. Bar chart of mean parasitoid wasps and winged aphids caught fortnightly on yellow sticky traps from 17 July to 17 October, 2018 in one hectare of canola at Northam, WA (N=10). Error bars indicate standard error of the mean.
Conclusion
Conserving and monitoring natural enemies of GPA in canola crops are important components of an integrated pest management program, especially given the insecticide resistance status of GPA and their ability to vector yield-limiting viruses (e.g. Turnip yellows virus). Predators, such as lacewing larvae and ladybird beetles, and parasitoid wasps, which have been observed naturally in WA crops, have the potential to provide useful levels of biological control for aphid pests such as GPA. It is evident that the ratio of natural enemies to GPA is a major factor in successful GPA population growth limitation or reduction. That is, the number of predators or parasitoid wasps relative to the numbers of GPA initially encountered by the natural enemies is a major determinant in whether the GPA will out-compete the effects of natural enemies and increase to damaging levels regardless, or whether GPA populations will stabilise or decrease over time and remain below damaging levels. Furthermore, GPA on the sentinel plants in both trials decreased while caged controls increased; this may be resultant of combined effects of parasitoid wasps and other natural enemies resident in the crop. This emphasises the importance of quick and reliable monitoring techniques for natural enemies and aphids in field crops. Yellow sticky traps which have been used reliably for winged aphid monitoring were shown to be useful for monitoring parasitoid wasps resident in the field over time. However, associations between the species and numbers of wasps on sticky traps and aphid parasitism rates (i.e. mummy counts) and population change require investigation in order to be able to reliably predict useful levels of biological control.
Acknowledgments
Thanks to Kanch Wickramarachchi for assistance with sticky trap assessments, GSARI Katanning and Rob deGruchy for trial sowing and maintenance, SARDI for provision of field cages and Thomas Heddle (SARDI) for rearing of the parasitoid wasps. This research is part of a national GRDC project being led by CSIRO and including colleagues at SARDI, NSW DPI, cesar and the University of Melbourne. The discussions and input into design by the project team are greatly appreciated.
GRDC Project Number: CSE00059
Reviewed by: Hazel Parry (CSIRO)
GRDC Project Code: CSE00059,
Was this page helpful?
YOUR FEEDBACK
