Sustaining our herbicide options into the future
Author: Christopher Preston, David Brunton, Peter Boutsalis, Tijana Petrovic, Mahima Krishnan and Gurjeet Gill (School of Agriculture, Food & Wine, University of Adelaide) | Date: 19 Feb 2019
Take home messages
- Resistance to pre-emergent herbicides in annual ryegrass is increasing and the resistance patterns are complex.
- Mixtures of pre-emergent herbicides can help manage resistant annual ryegrass populations when coupled with seed set control tactics.
- Resistance to 2,4-D in broadleaf weeds is increasing. Low level resistance is easy to control, but high-level resistance requires additional practices.
Resistance to pre-emergent herbicides
The increasing incidence of resistance to post-emergent herbicides in annual ryegrass has meant a much greater dependence on pre-emergent herbicides for control of this weed. This has the unwanted consequence of selecting for resistance to pre-emergent herbicides. In recent years, resistance to pre-emergent herbicides has been increasing in annual ryegrass populations. Resistance to trifluralin is widespread in South Australia (SA) and western Victoria. The extent of resistance to trifluralin is increasing in NSW.
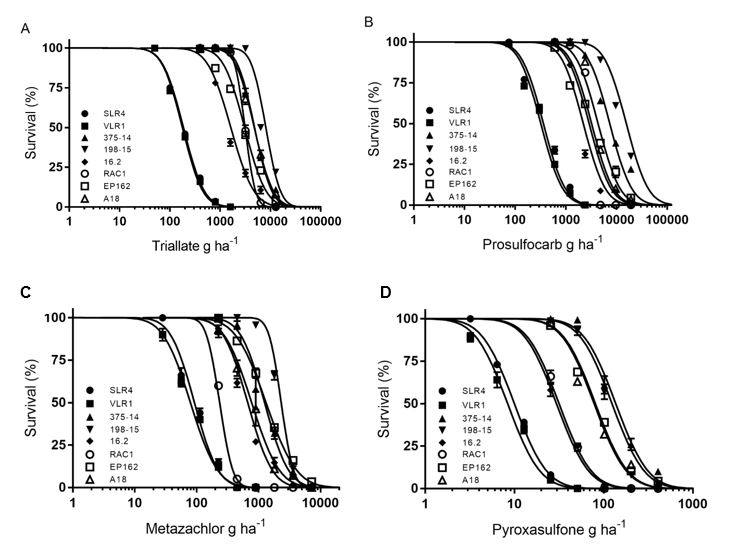
More recently, failures of Avadex Xtra® (triallate - Group J), Boxer Gold® (prosulfocarb – Group J + S-metolachlor - Group K) and Butisan® (metazachlor – Group K) to control annual ryegrass have been reported from the field. The problem is that some of these populations have resistance to numerous pre-emergent herbicides. Several of these populations have moderate levels of resistance to the Group J herbicides and some cross-resistance to the Group K herbicides. We tested six different resistant populations from SA and NSW. The levels of resistance to triallate and prosulfocarb were similar in these populations, but there were varying levels of resistance to the Group K herbicides metazachlor and pyroxasulfone (Figure 1).
Figure 1. Response of two susceptible (SLR4 and VLR1) and six resistant annual ryegrass populations to various pre-emergent herbicides. (A) Triallate, (B) Prosulfocarb, (C) Metazachlor and (D) Pyroxasulfone.
Options for controlling annual ryegrass resistant to the pre-emergent herbicides
Two field trials were conducted at Arthurton and Paskeville, SA, in wheat in 2018 to explore opportunities for managing resistance to pre-emergent herbicides. Both sites had weed populations with high resistance to trifluralin, moderate resistance to the Group J herbicides and low resistance to the Group K herbicides. Growing conditions during 2018 were challenging with below average rainfall between April to July, resulting in lower than normal activation of pre-emergent herbicides.
In the field trials, trifluralin was relatively ineffective at reducing annual ryegrass at both sites and did not reduce annual ryegrass spike production, consistent with the high level of resistance to trifluralin present (Table 2). Triallate (Avadex Xtra®) alone reduced weed density at Arthurton, but not at Paskeville. Prosulfocarb, as either Arcade® or Boxer Gold®, provided higher levels of control and reduced annual ryegrass seed set. Pyroxasulfone (Sakura®) was less effective at reducing annual ryegrass numbers, particularly at Paskeville. This was probably the result of the dry seasonal conditions, as this herbicide requires more moisture to activate. Sakura® tended to be better at reducing annual ryegrass seed set.
Despite resistance to all of the herbicides used being present at the two sites, mixtures of pre-emergent herbicides were more effective at controlling annual ryegrass and reducing seed set (Table 1) than using single herbicides alone. Mixtures of pre-emergent herbicides can be useful where annual ryegrass populations are high or where conditions for pre-emergent herbicides to work are poor. These trials show that where moderate or low resistance to pre-emergent herbicides is present, mixtures can also help control resistant populations.
Table 1. Control of herbicide resistant annual ryegrass with pre-emergent herbicide options at Arthurton and Paskeville in 2018. All herbicides were applied incorporated by sowing (IBS) prior to sowing. Weed density was determined eight weeks after sowing (WAS) and spike density at 18 WAS. Values with different letters in each column were significantly different.
Treatments | Rate (g a.i./ha) | Weed density (plants/m2) | Spike density (spikes/m2) | ||
|---|---|---|---|---|---|
Paskeville | Arthurton | Paskeville | Arthurton | ||
Untreated | 688 f | 518 i | 514 g | 294 f | |
Triallate | 1500 | 609 f | 191 f | 318 f | 223 d |
Trifluralin | 1200 | 832 g | 388 h | 507 g | 295 f |
Trifluralin + triallate | 1200 + 1500 | 601 ef | 282 g | 339 f | 279 f |
Prosulfocarb | 2400 | 105 a | 119 bc | 190 de | 256 e |
Prosulfocarb + S-metolachlor | 2000 + 300 | 160 b | 136 cd | 159 bc | 213 d |
Pyroxasulfone | 100 | 510 e | 171 e | 204 e | 90 a |
Prosulfocarb + S-metolachlor + triallate | 2000 + 300 + 1500 | 262 d | 173 e | 168 cd | 168 c |
Prosulfocarb + triallate | 2400 + 1500 | 259 cd | 136 cd | 107 a | 178 c |
Pyroxasulfone + triallate | 100 + 1500 | 162 b | 143 d | 131 ab | 128 ab |
Pyroxasulfone + prosulfocarb + S-metolachlor | 100 + 2000 + 300 | 106 a | 102 a | 165 cd | 85 a |
Resistance to herbicides in broadleaf weeds
Another problem that is increasing in prominence is resistance to 2,4-D in broadleaf weeds. With increasing resistance to Group B herbicides in broadleaf weeds, 2,4-D and the other Group I herbicides have been used more extensively for the management of troublesome broadleaf weeds in cereal crops and fallows. This extra selection pressure is resulting in resistance to Group I herbicides.
The major weeds of concern are wild radish, Indian hedge mustard and common sowthistle. Wild radish with resistance to Group I herbicides is present in cropping regions of NSW, Victoria, Tasmania and SA. Resistant Indian hedge mustard mostly occurs in SA with a few isolated populations in Victoria. Resistant sowthistle is also mostly occurring in SA with isolated populations in Victoria and NSW.
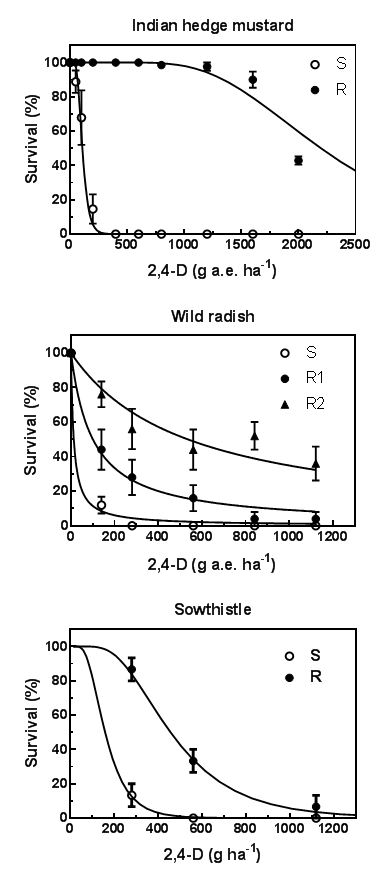
Resistance to the Group I herbicides appears to come in at least two forms that are probably related to different resistance mechanisms. There is a high-level resistance form, seen commonly in Indian hedge mustard and in some wild radish populations (Figure 2), and a low-level resistance form, seen in sowthistle and most wild radish populations. The low-level resistant individuals typically show strong symptoms of stem and leaf twisting and swelling, but the plants do not die and start to re-grow after 14 days or so. The high-level resistant individuals show no, or slight symptoms of the herbicide and recover within a few days to look normal.
These different forms of resistance can have consequences for management with herbicides. Where the level of resistance is low, mixtures of 2,4-D with other herbicides coupled with crop competition can provide effective control. Where the level of resistance is high, other practices will have to be used. There are alternative mode of action herbicides from Groups H and G that can be effective in mixtures on these species. Harvest weed seed management strategies can work for wild radish and Indian hedge mustard, but will not be very effective for sowthistle. Wild radish being an outcrossing species can accumulate additional resistance mechanisms and may become more resistant to 2,4-D over time. For wild radish management, a two-spray strategy of a contact herbicide early post-emergence (e.g. Velocity® or Talinor®) followed by a more systemic product (e.g. Flight® or Triathlon®) typically works well.
Figure 2. Response to 2,4-D of susceptible (S) and resistant (R) populations of Indian hedge mustard with high level resistance to 2,4-D (top), wild radish with high level and low-level resistance (middle), and sowthistle with low level resistance (bottom).
Useful resources
http://sciences.adelaide.edu.au/agriculture-food-wine/system/files/docs/2016-wild-radish.pdf
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC – the author would like to thank them for their continued support. We would like to also thank Chris Davey of YPAg for his assistance with the pre-emergent field trial.
Contact details
Christopher Preston
School of Agriculture, Food & Wine, University of Adelaide
0488 404 120
christopher.preston@adelaide.edu.au
Was this page helpful?
YOUR FEEDBACK