Towards prediction of diamondback moth risk in canola - new insights into ecology and resistance management
Author: Kym D Perry and Greg J Baker. (South Australian Research & Development Institute) (School of Agriculture Food and Wine, University of Adelaide) | Date: 12 Feb 2019
Take home messages
- The seasonal risk of spring diamondback moth outbreaks in canola starts in autumn, driven by pre-season rainfall patterns.
- Brassicaceous weeds allow insecticide-resistant diamondback moth to persist during summer in canola-growing areas of South Australia (SA).
- A new insecticide resistance management strategy is available for the diamondback moth.
- A ‘cryptic’ diamondback moth (Plutella Australiana) species poses little threat to Australian canola.
Background
The diamondback moth, Plutella xylostella, is the principal spring pest of Australian canola (Furlong 2008). This species is highly mobile and colonises Australian canola crops consistently each season, leading to intermittent outbreaks. Under favourable seasonal conditions, larval populations in canola increase to extremely high densities in spring, resulting in the destruction of maturing plants and high yield losses. Growers are reliant on insecticides to manage outbreaks; however, insecticide resistance is widespread in Australian diamondback moth populations (Baker 2015). The few insecticide products currently available and effective for diamondback moth control in canola have alternative use patterns and can be in short supply during outbreaks. Enhancing growers’ capacity to manage diamondback moth requires (1) better forewarning of seasonal outbreak risk and (2) the development and industry-wide adoption of effective insecticide resistance management strategies. Both objectives require a thorough understanding of the pest’s ecology.
Recent PhD research by Kym Perry (University of Adelaide, SARDI) in collaboration with SARDI colleagues (Greg Baker, Kevin Powis, and Joanne Kent) and many agronomists, with investment support from GRDC and the University of Adelaide, has revealed new insights into diamondback ecology and resistance management in Australian canola. The research contributes to industry’s goal of improved pest risk prediction.
Here, the new diamondback moth research and management guidelines are summarised within the following subject headings:
- The likely origins of diamondback moth that seasonally colonise canola in South Australia.
- The role of brassicaceous weeds and pre-season weather in driving crop colonisation patterns and seasonal diamondback moth risk.
- The importance of a ‘cryptic’ diamondback moth to Australian canola growers.
- New insecticide resistance management guidelines for the diamondback moth.
Cryptic diamondback moth species
In 2013, Canadian researchers reported the discovery of a ‘cryptic’ species (genetically distinct but looks identical) of diamondback moth in Australia, which they named Plutella australiana (Landry and Hebert 2013). In 2014 to 2017, SARDI conducted thorough investigations into its biology, distribution and pest status in Australian Brassica crops, with GRDC investment support.
These investigations found:
- Both Plutella species occur on wild brassicas and canola crops throughout southern and western Australia, but the diamondback moth, P. xylostella, is the predominant species. Of the >1400 individuals collected from brassicas in 2014 to 2015 and screened using molecular assays, 90% were P. xylostella (Figure 1).
- Insecticide bioassays revealed that P. australiana was 19 to 306 fold more susceptible than P. xylostella to three insecticides commonly used for diamondback mothcontrol: alpha-cypermethrin (synthetic pyrethroid (SP), Group 3A), emamectin benzoate (Group 6), spinetoram (Group 5), and soon to become available chlorantraniliprole (Group 28). Unlike the diamondback moth; P. xylostella, no evidence was found that P. australiana evolves insecticide resistance.
- While both species attack brassicaceous plants, a strong genetic difference between the species was found, as well as clear differences in host preferences and performance on different host plants.
Although P. australiana does attack canola, it is far less abundant than P. xylostella and should be easily controlled using commercial insecticides. Consequently, P. australiana is considered to pose little threat to Australian canola yields.
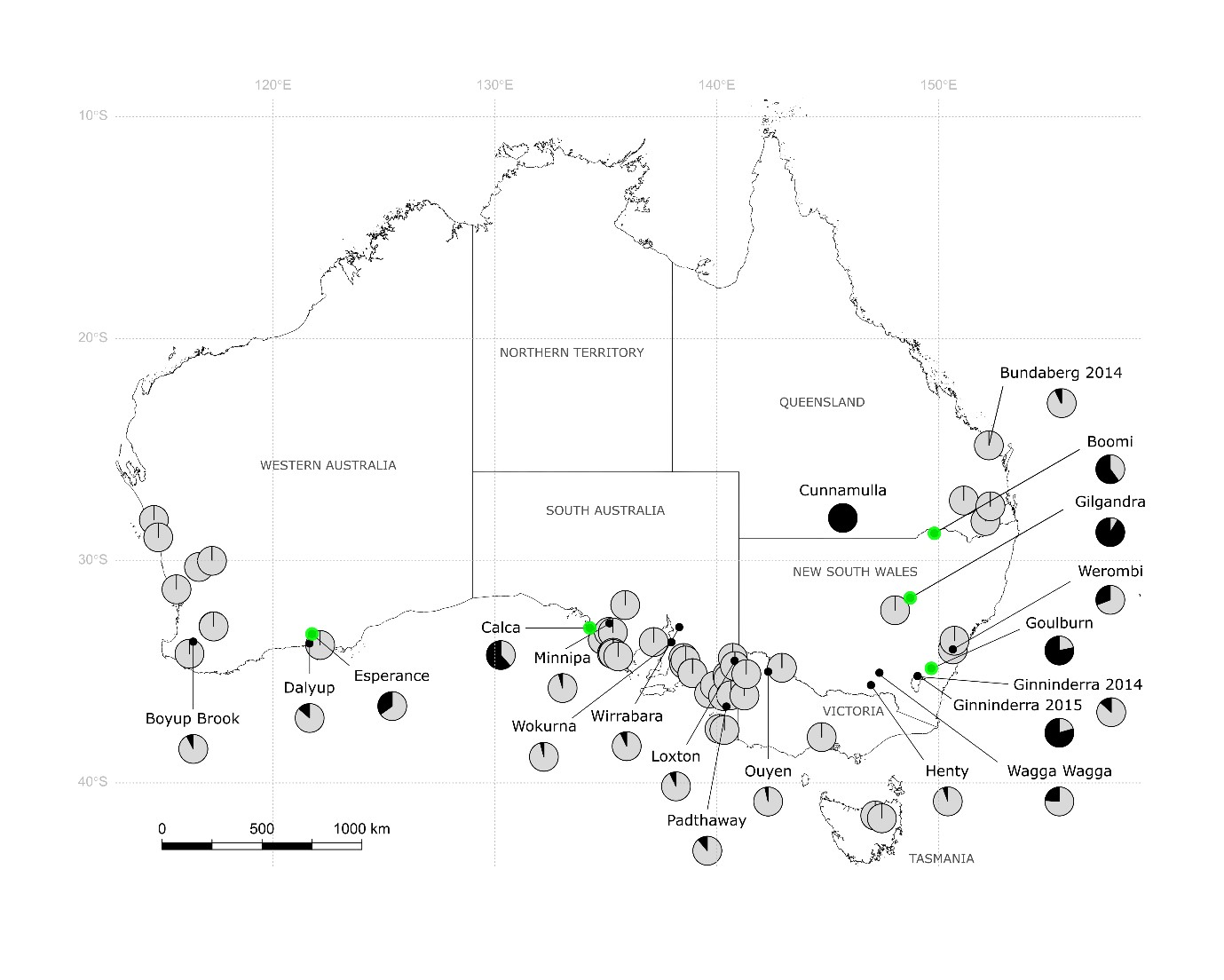
Figure 1. The geographic distribution of P. xylostella (light grey) and P. australiana (black) in Plutella collections from brassicaceous host plants during 2014 and 2015. Pie diagrams show the relative proportion of each species at each location, and overlapped pies represent locations with 100% P. xylostella (Source: Perry et al 2018 BMC Evolutionary Biology).
The seasonal colonisation of canola by the diamondback moth - where does it come from?
For highly mobile insect pests, developing a capacity to predict seasonal pest pressure in crops requires sound knowledge of their arrival timing and origins. The origins of diamondback moth that seasonally invade Australian winter canola crops have been uncertain.
Field studies were conducted at a regional scale in SA over three years from 2014 to 2016 to determine (1) the presence of diamondback moth on local green bridge between growing seasons, and (2) the arrival timing of diamondback moth in canola crops. Each season, potential host plants (wild brassicas) were sampled for diamondback mothduring the autumn pre-season, focusing on Eyre Peninsula (the South East SA was included in 2015), then a network of sentinel canola fields (approximately30 per year) was monitored across SA and Western Victoria to detect diamondback moth arrival. Agronomists each monitored one to two canola fields approximately weekly. All sampling involved trapping moths using pheromone traps and sampling plants directly for larvae (Figure 2). Models were run using local climate data for each site to predict the date of first oviposition (egg lay, signalising colonisation) in each canola crop, and the suitability of the site for earlier build-up of diamondback moth populations. This allowed inferences as to whether the moths came from the local areas or from further away.
These investigations found:
- Summer-active wild brassicas support widespread populations of insecticide-resistant diamondback moth over summer. Their resistance profiles were near-identical to populations from canola (Baker, 2015), indicating frequent movement between crops and weeds. On Eyre Peninsula, the key brassicas harbouring diamondback moth were Lincoln weed (Diplotaxis tenuifolia), dog weed (D. muralis) and sea rocket (Cakile maritima), the latter being a species occurring in coastal sand dunes. In the south east of SA, Brassica forage crops provide an additional summer host.
- Most canola crops across SA and Western Victoria were colonised during May and June, soon after crop emergence. This indicates that spring outbreaks can be caused by a season-long increase of populations in crops and not necessarily migrations (unlike Helicoverpa, where migrations are the major factor).
- Across seasons, pre-season rainfall had a strong influence on populations of wild brassicas and diamondback moth in autumn and the subsequent colonisation pattern in canola. In 2014 and 2016, rain between February and April promoted weed growth and diamondback moth populations, then canola crops were colonised soon after germination. By contrast, dry conditions in 2015 severely limited pre-season host plant availability, leading to scarce diamondback moth, and canola crops were colonised (on average) later in the season.
- These data along with climate niche modelling and light trapping data (not shown) suggest diamondback moth that seasonally colonise canola originate primarily from local summer brassicas in canola-growing areas.
In this region, early season diamondback moth pressure in canola appears largely driven by local pre-season rainfall and host plant vegetation response. Future research should explore colonisation patterns in other regions and determine the extent to which autumn populations are a pre-cursor to spring populations (i.e. and hence useful for forecasting).
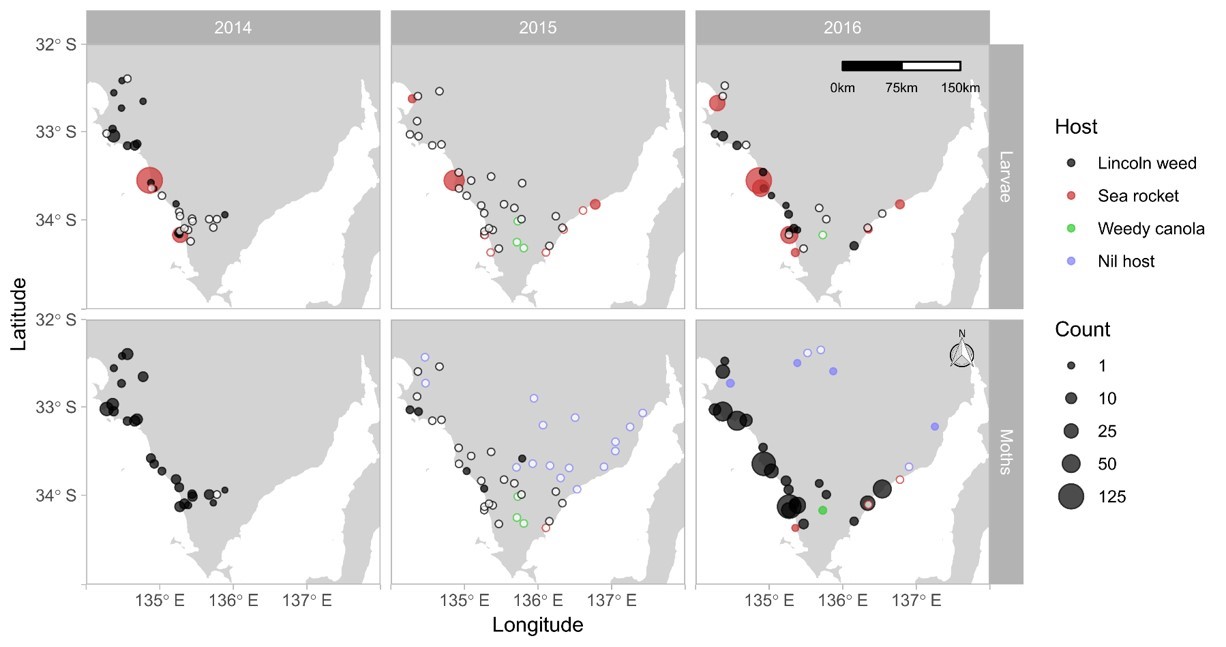
Figure 2. Abundance of diamondback moth in pheromone traps (lower panels, moths per trap over 4 weeks) or larvae (upper panels, larvae per 20 plant samples) associated with wild brassicaceous plants during autumn surveys on Eyre Peninsula in March and April from 2014 to 2016. Circle size is scaled to abundance; open circles indicate absence at a sampled location; filled and open circles are colour-coded by host plant (Source: Perry KD, 2018).
Insecticide resistance management for diamondback moth
Current insecticide resistance status
Five chemical sub-groups are registered to control diamondback moth in Australian canola: synthetic pyrethroids (Group 3A); organophosphates (Group 1B); spinosyns (Group 5); avermectins (Group 6); and Bacillus thuringiensis (Group 11A). Carbamates (Group 1A) are also registered for use in canola in Western Australia. Of these, only two synthetic insecticides (Group 5 spinetoram and Group 6 emamectin benzoate) and several Bacillus thuringiensis var. kurstaki products (BtK, Group 11A) are capable of reliably providing efficacious control of diamondback moth. Resistance to older synthetic insecticides (e.g. SPs/group 3A, and OPs/group 1B) is widespread in diamondback moth populations throughout all Australian canola production areas, rendering these chemicals partially or totally ineffective for diamondback moth control. There is generally no geographic pattern to diamondback moth resistance profiles in Australian canola production areas – for a given insecticide, resistance ratios tend to be similar across canola production regions, and between canola crops and weeds within a region, reflecting a high degree of gene flow among diamondback moth populations.
Resistance screening of diamondback moth populations from canola since 2008 has detected increasing tolerance to the newer chemistries available (emamectin benzoate, spinetoram) or soon to become available (Group 28, cyantraniliprole, cyclaniliprole) for diamondback moth control in Australian canola (Baker 2015). Interestingly, resistance screening since 2015 has detected a decline or stabilisation in resistance levels to all tested insecticides (Table 1). The reasons for this decline are unclear; however, a low incidence of diamondback moth outbreaks during the past three to four years may have contributed to reduced usage and hence selection pressure for these insecticides. While these results are encouraging, increased insecticide use is expected to quickly increase resistance levels.
Table 1. Resistance ratios at LC50 to four insecticides tested on diamondback moth populations collected from canola since 2015-2018 (project DAS00155) compared to 2008-2015 (project DAS00094), with the population screened in parentheses.
Chemical | Mean resistance ratio (RR) at LC50 | |
|---|---|---|
2008-2015 | 2015-2018 | |
Alpha-cypermethrin (Group 3A) | 141.2 (n=39) | 79.4 (n=3) |
Emamectin benzoate (Group 6A) | 6 (n=38) | 2.7 (n=28) |
Spinetoram (Group 5) | 1.8 (n=21) | 1.6 (n=1) |
Chlorantraniliprole (Group 28) | 13.1 (n=39) | 7.6 (n=28) |
(n = number of populations)
New Insecticide resistance management strategy for the diamondback moth
A new insecticide resistance management (IRM) strategy for diamondback moth has been developed in conjunction with the Grains Pest Advisory Committee (GPAC) and agronomists and endorsed by CropLife Australia. The strategy was launched in June 2017 on the GRDC and CropLife Australia websites (see Useful resources section within this paper). To maximise the effective life of newer diamondback moth chemistries, canola growers and their advisers are recommended to follow the management guidelines in the new IRM strategy (Figure 3). The strategy aims to reduce pressure for resistance selection on consecutive generations of diamondback moth through use of integrated management and rotation of effective insecticide classes.
Key points from the strategy are:
- Only spray when necessary (use a threshold-based approach).
- Avoid using SPs (Group 3A) for diamondback moth control in canola.
- Consider using Bacillus thuringiensis (BtK) in rotation strategies.
- Rotate insecticides – avoid using the same product within the same season or in consecutive seasons.
- Adjust rotations when controlling diamondback moth and Helicoverpa together (Figure 3).
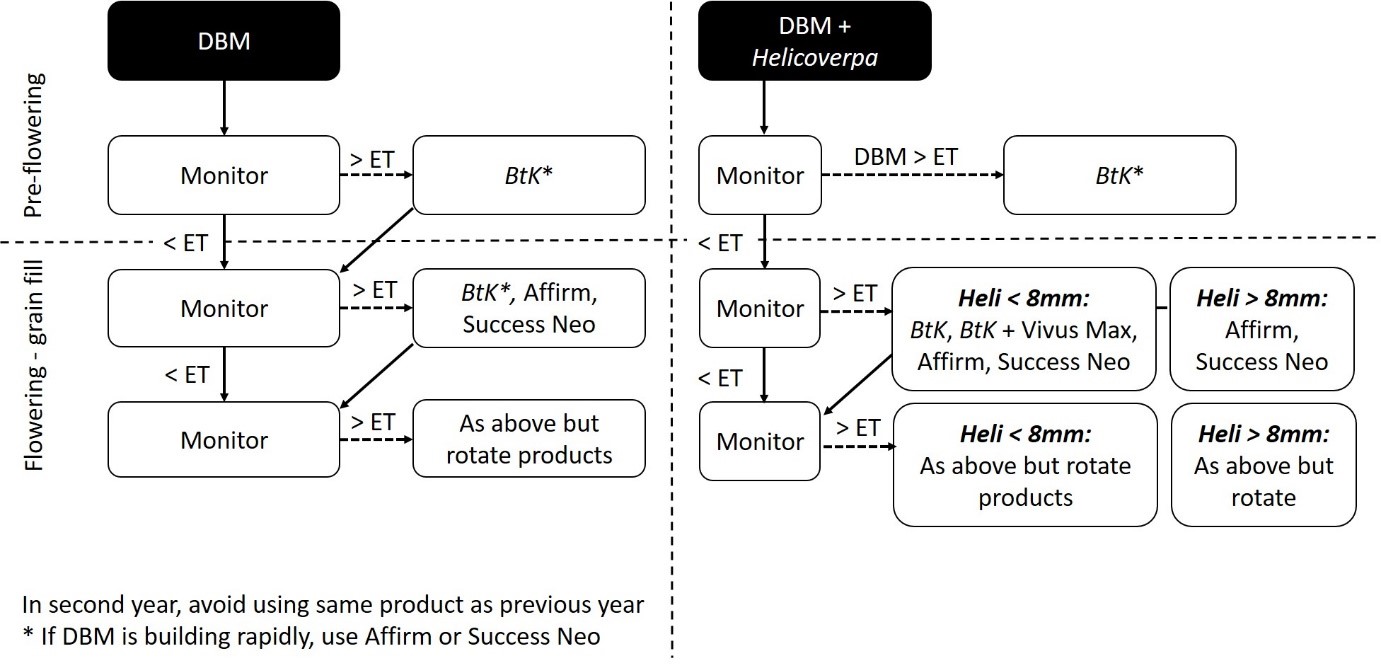
Figure 3. Schematic summary of the new Insecticide Resistance Management Strategy (RMS) for diamondback moth (summarised from Baker et al 2017).
Conclusion
Predicting pest pressure remains a major goal for pest management but requires deep ecological understanding at a species level. The approach used here, involving collaboration between researchers and agronomists across SA and Western Victoria in data gathering, made it possible to conduct a study of a mobile insect pest at a regional geographic scale. This approach provides a template for ecological research into other pests with similar mobility and for which prediction would be useful, such as aphids.
Here, thorough research into the diamondback has provided important insights into factors driving its seasonal abundance in SA. Specifically, the availability of weedy brassicas driven by pre-season rainfall contributes to early season diamondback moth pressure in canola. Brassica weeds also allow insecticide resistance to persist in local diamondback moth populations. While achieving area-wide weed management would be ideal, it may be more achievable in the short term to focus attention on improving forecasts of seasonal outbreak risk based on pre-season climate/green bridge information. To achieve this, future studies over longer timeframes and in other regions are required.
Growers rely on access to effective chemistries to manage diamondback moth outbreaks. To maximise the life of newer chemistry, it is recommended that all growers and advisers adopt the new IRM guidelines for the diamondback moth. Industry-wide adherence to the strategy will maximise its effectiveness.
Useful resources
Resistance management strategy for diamondback moth in Australian canola. https://grdc.com.au/fs-resistancestrategydiamondbackmoth
Science behind the RMS for diamondback moth (Plutella xylostella) in Australian canola crops, NIRM https://ipmguidelinesforgrains.com.au/important/uploads/Science-Behind-the-Resistance-Management-Strategy-for-DBM-in-canola.pdf
Diamondback moth factsheet. GRDC, www.grdc.com.au/GRDC-FS-DBM
References
Baker, GJ (2015). Improving management of diamondback moth in Australian canola: Final report for GRDC. Technical report, South Australian Research and Development Institute.
Baker, G., Umina, P., Miles, M., Schellhorn, N., Hoffmann, A., Edwards, O., McDonald, G., Powles, S. and Cornwell, G. (2017). Science behind the RMS for diamondback moth (Plutella xylostella) in Australian canola crops, NIRM https://ipmguidelinesforgrains.com.au/important/uploads/Science-Behind-the-Resistance-Management-Strategy-for-DBM-in-canola.pdf
Furlong MJ, Spafford H, Ridland PM, Endersby, NM, Edwards, OR, Baker GJ, Keller MA and Paull CA (2008). Ecology of diamondback moth in Australian canola: Landscape perspectives and the implications for management. Australian Journal of Experimental Agriculture, 48(12):1494–1505.
Landry JF and Hebert PDN (2013). Plutella australiana (Lepidoptera, Plutellidae), an overlooked diamondback moth revealed by DNA barcodes. ZooKeys, 327:43–63.
Perry KD, Baker GJ, Powis KJ, Kent JK, Ward CM, and Baxter SW.
(2018a). Cryptic Plutella species show deep divergence despite the capacity to hybridize.
BMC Evolutionary Biology, 18:77. https://doi.org/10.1186/s12862-018-1183-4
Acknowledgements
The research undertaken as part of this project was made possible through funding provided by the University of Adelaide and by the significant contributions of growers and agronomists through both trial cooperation and the support of the GRDC. The authors would like to thank them for their continued support.
KD Perry gratefully acknowledges the many colleagues who participated in regional monitoring of the diamondback moth:
Adam Hancock, Adam Pearce, Adam Quade, Alan Lord, Alf Weaver, Andrew McMahen, Andrew Sharpe, Andy Bates, Andy Ryland, Ben Farmer, Brad Bennett, Brenton Spriggs, Cherylynn Dreckow, Craig Davis, Chris Davey, Chris Pearce, Chris Teague, Cindy Martin, Clint McEvoy, Craig James, Craig Wissell, Darren Pech, Dustin Berryman, Dylan Fox, Emma Leonard, Grant Hudson, Guy Westmore, Hayden Whitwell, Ian Koch, James McKee, Jenny Hazelgrove, Jessica Smith, Joanne Holloway, Josh Andrews, Josh Hollitt, Karina Bennett, Kaye Zadow, Laura Archer, Leigh Davis, Brenton Spriggs, Levon Cookson, Lisa Ohlson, Louise Flohr, Lyn Dohle, Luke Wilkins, Matthew Howell, Melina Miles, Michael Camac, Michael Collins, Monica Field, Michael Hind, Natalie Allen, Nigel Myers, Patrick Redden, Paul Ackland, Peter Cole, Peter Ellison, Marieke Ellison, Peter Gregg, Peter Mangano, Richard Saunders, Sally MacLeod, Samuel Boehm, Sarina Macfadyen, Sarah Meyer, Scott Hutchings, Simon Honner, Steve Hein, Steve Richmond, Stewart Learmonth, Steve Manning, Sarah Noack, Thomas Cooper, Tim Richardson.
Contact details
Kym Perry
SARDI Entomology Unit
Waite Campus, Urrbrae SA 5064
8429 0738
kym.perry@sa.gov.au
@PestFactsSARDI
Greg Baker
SARDI Entomology Unit
Waite Campus, Urrbrae SA 5064
8429 0933
greg.baker@sa.gov.au
Was this page helpful?
YOUR FEEDBACK
