Use of elemental sulphur coated phosphorus fertiliser for canola production
Author: Geoff Anderson, DPIRD | Date: 25 Feb 2019
Key Messages
- Low levels of elemental sulphur (ES) oxidising bacteria and dry seasonal conditions during May and June resulted in ES coated phosphorus fertiliser not supplying sufficient S for canola production so sufficient sulphate sulphur (SS) needs to be applied.
- Application of SS fertiliser greater than 10 kg S/ha resulted in reduced canola biomass production due to reduced molybdenum (Mo) uptake by canola. However, this reduced biomass growth did not impact on grain yield.
Aims
This paper aims to examine the effectiveness of ES coated high phosphorus analysis fertiliser granules as an S source compared to applying SS as ammonium sulphate. This research is important because growers prefer to use fertiliser products like MAP, DAP and urea to supply phosphorus and nitrogen to their crops which have no S in the case of urea and 1.5-1.7% S in the case of MAP and DAP. Using only this approach growers are likely to run into S deficiency (Anderson et al. 2016).
The S content of MAP and DAP fertiliser can be increased using a new manufacturing process Shell Thiogro (Shell Thiogro) or by coating fertiliser granules with S. The Thiogro process produces a range of fertiliser products called S enhanced fertilisers. These fertilisers can be manufactured with selected ES and SS contents. Having both forms with the fertiliser provides SS, which is immediately available to the plant and ES which provides a slow release form. ES is 100% S and when mixed with other fertiliser products less effect on the P and N concentration of the fertiliser compared to SS. The S content of the fertiliser can be increased by coating ES onto the fertiliser granules (Pitala and Blair 2017). Having ES in the fertiliser instead of SS reduces the handling costs associated with applying P fertiliser while maintaining the S content of the fertiliser. Fertilisers with increased S levels produced by both of these processes were used in this experiment.
Method
The location of the experiment was 7.5 km west of Moora (30°37'14.94"S 115°54'28.77"E) on yellow deep sand. Soil samples were collected in July to measure the soil chemical properties (Table 1). The samples were collected using a 0-10 cm and 0-30 cm pogo. The soil had a low KCl40-S soil S test of 4.2 mg S/kg in 0-10 cm, 3.2 mg S/kg in 0-30 cm. Soil DNA extraction and quantitative PCR measurement of soxB and 16S rRNA gene abundance, as indicators for S-oxidising bacteria and total bacteria, were measured on soil samples collected in September and December (Zhao et al. 2017).
Table 1. Summary of measured soil chemical properties
Depth | Inorganic N | Colwell P | PBI | KCl 40 S | Colwell K | Carbon | pH (CaCl2) | Al (CaCl2) |
|---|---|---|---|---|---|---|---|---|
cm | (kg/ha) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (%) | (mg/kg) | |
0-10 pogo | 60 | 25 | 19 | 4.2 | 37 | 1.0 | 6.1 | 0.4 |
0-30 pogo | 81 | 20 | 20 | 3.2 | 31 | 0.7 | 5.5 | 0.5 |
In this experiment the effectiveness of the coated ES was compared with SS or ammonium sulphate by applying these S sources at the same S rates for three types of phosphate fertilisers (Table 2). To achieve this objective the experiment consisted of thirty treatments; five S rate treatments 0, 2.5, 5.0, 10.0 and 15.0 kg S/ha, two S sources coated ES or SS and three P fertilisers. The experimental design was a randomised block with three replicates.
The P fertilisers used were, firstly, triple superphosphate (TSP) which has a sulphate content of 1%. Secondly, a mono-ammonium phosphate (MAP)-based fertiliser (MAPSZC®) with an S content of 5% of which 3.7% is in the form of ES with a particle size of less than 30 µm and 1.3% in the form of sulphate. Thirdly, a second MAP-based fertiliser (Granulock SS®) with 12% S of which 8% is in the form of ES particle size of less than 30 µm and 4% in the form of SS. Both MAPSZC® and Granulock SS® are S enhanced fertiliser with both the ES and SS added during manufacture using the Thiogro process.
ES was coated on to these P fertilisers to increase the S content of the fertilisers. The experiment used an ES product supplied by Manutec called dusting S which had a mean particle size of 50 µm. The ES was coated onto the fertiliser granules using a 14-inch rotary seed coater (Brace Works, Canada) with calcium lignosulfonate used as the binding agent (Pitala and Blair 2017). The amount of ES coated onto the P fertilisers granules was calculated to give S rates of 0, 2.5, 5.0, 10, and 15 kg S/ha when the P fertiliser was applied at a rate of 7.5 kg P/ha (Table 2). Corresponding rates of Ammonium sulphate was applied at rates to give corresponding rates of additional S as the ES coating (i.e. 0, 2.5, 5.0, 10, and 15 kg S/ha), so that a direct comparison could be made between S applied in the form of ES and SS.
The P fertilisers TSP, MAPSZC® and Granulock SS® differ in S content. Hence, when the fertilisers were applied at the application rate of 7.5 kg P/ha the application of fertiliser S was 0.4 kg/ha when TSP was applied, 2.1 kg/ha when MAPSZC® was applied and 5.3 kg/ha when for Granulock SS® (Table 2). When combined with the additional S treatments, this gave a range of total S applied.
Table 2. Additional S rate treatments (kg S/ha) when applied as ES coated onto the fertiliser granule or applied as SS in the form ammonium sulphate. Fertiliser S (kg S/ha) applied in the form of SS and ES applied due to the difference in P fertiliser S composition. Total S (kg S/ha) applied is the sum of the additional S rate treatments, fertiliser SS and fertiliser ES.
No | P source | Additional S applied as ES or SS (kg S/ha) | Fertiliser SS (kg S/ha | Fertiliser ES (kg S/ha) | Total S applied (kg S/ha) |
|---|---|---|---|---|---|
1, 16 | TSP | 0.0 | 0.4 | 0.0 | 0.4 |
2, 17 | TSP | 2.5 | 0.4 | 0.0 | 2.8 |
3, 18 | TSP | 5.0 | 0.4 | 0.0 | 5.3 |
4, 19 | TSP | 10.0 | 0.4 | 0.0 | 10.4 |
5, 20 | TSP | 15.0 | 0.4 | 0.0 | 15.4 |
6, 21 | MAPSZC® | 0.0 | 0.7 | 1.4 | 2.1 |
7, 22 | MAPSZC® | 2.5 | 0.7 | 1.4 | 4.6 |
8, 23 | MAPSZC® | 5.1 | 0.7 | 1.4 | 7.2 |
9, 24 | MAPSZC® | 10.0 | 0.7 | 1.4 | 12.1 |
10, 25 | MAPSZC® | 15.0 | 0.7 | 1.4 | 17.1 |
11, 26 | GranulockSS® | 0.0 | 1.8 | 3.4 | 5.2 |
12, 27 | GranulockSS® | 2.5 | 1.8 | 3.4 | 7.7 |
13, 28 | GranulockSS® | 5.1 | 1.8 | 3.4 | 10.3 |
14,,29 | GranulockSS® | 10.0 | 1.8 | 3.4 | 15.2 |
15, 30 | GranulockSS® | 15.0 | 1.8 | 3.4 | 20.2 |
Nitrogen fertiliser applied at seeding was 19 kg N/ha. If the fertiliser treatment resulted in a lower N rate, additional N was applied in the form of urea to bring the seeding N rate to 19 kg N/ha. Other basal seeding fertiliser applications were 70 kg K/ha in the form of KCl (applied due to the low soil K test), 0.3 kg Zn/ha and 0.9 kg Cu/ha. An additional application of 59 kg N/ha in the form of UAN was applied to all treatments on the 22 August 2017.
The site was sown dry to canola (cv Bonito) at 5 kg/ha on 8 May 2017 with germination occurring following rainfall of 18 mm over the period 16-22 May 2017. Biomass cuts (50 cm by two rows) were made on 11 August 2017 and analysed for nutrient content. Plot harvest to derive grain yield (t/ha) occurred on the 2 November 2017. Growing season rainfall at the experimental site was (287 mm) which was lower than the long-term median of 362 mm due to low May (23 mm) and June (49 mm) rainfall. The low May-July rainfall resulted in a low leaching intensity for the site in 2017.
Analyses of variance were conducted using treatments effects S source (ES vs SS) by fertiliser source (TSP vs MAPSZC® vs GranulockSS®) by S rate (2.5, 5.0, 10, and 15 kg S/ha) in Genstat®. Only treatment main effects and interactions determined to be significant at P=0.05 are reported.
The McCaskill and Blair (1989) and Degryse et al. (2016) models were included in a daily water balance model (Anderson et al. 2011) and linked to meteorology data obtained from DPIRD meteorology site located 5 km to the north-west of the experimental site. These models were used to estimate the rate of ES oxidation for the 2016 and 2017 growing seasons.
Results
Populations of S-oxidising bacteria (SOB) in the soil were 2.7 x 106/g dry soil while total bacterial populations were 1.2 x 109/g dry soil. There was no significant difference in the amount of S-oxidising bacteria between the control and elemental S applied treatments and between September and December sampling times. The population of S-oxidising bacteria as a percentage of total bacteria was less than 0.5 % (0.17 to 0.31%).
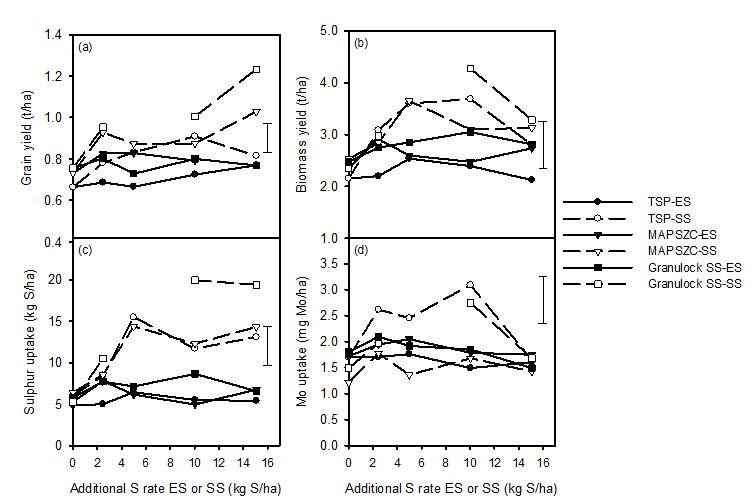
Applying additional S as ES or SS produced differences within the response curves of grain yield, biomass yield, S uptake and Mo uptake (Figure 1) due to the variation of the S content of the P fertiliser. For example GranulockSS® generally provided the highest response curve due it containing the highest amount of fertiliser S of 5.2 kg S/ha.
Sulphur applied in the form of sulphate produced a 16% higher grain yield (t/ha) than S applied in the form of ES, S source main effect (Figure 1a). GranulockSS® fertiliser produced 12% higher grain yield than TSP fertiliser, fertiliser main effect. Sulphur in the form of sulphate increased grain yield by 24% while S in the form of ES resulted in an 8% increase in grain yield (rate by S source interaction). The combination of 15 kg S/ha sulphate fertiliser application plus Granulock SS® (total S applied of 20.2 kg/ha) produced the highest grain yield which was 48% higher than the TSP without additional applied S (Table 1, Treatment no 1 and 16).
Figure 1. (a) Canola grain yield (t/ha), (b) canola biomass (t/ha), (c) S uptake by canola (kgS/ha) and (d) Mo uptake by canola (mg Mo/kg) for the P fertilisers TSP (l), MAPSZC® () and Granulock SS® (q) with additional S supplied in the form of coated ES or SS. Error bars are the LSD at P=0.05 for the S x P fertiliser x S rate interaction. Missing data for GranulockSS®-SS response curve was due to an experimental error with the 5 kgS/ha rate.
Sulphur applied in the form of sulphate produced a 17% higher biomass yield (t/ha) than S applied in the form of ES, sulphate source main effect (Figure 1b). GranulockSS® fertiliser produced similar biomass yield to TSP fertiliser with a difference of only 7%, P fertiliser main effect. Maximum biomass yield occurred at sulphate rate of 10 kg S/ha which produced 23% higher biomass yield than the control. Application of 10 kg S/ha increased biomass yield by 16-37% when sulphate was applied compared to a 19-28% increase when ES was applied. Application of SS at 15 kg S/ha resulted in a 14-24% reduction in canola biomass for the three P fertilisers compared to SS applied at 10 kg S/ha.
Sulphur applied in the form of SS resulted in a 42% higher S uptake by canola (kg S/ha) than S applied in the form of ES, sulphate source main effect (Figure 1c). GranulockSS® fertiliser produced a similar S uptake to MAPSZC® fertiliser with a difference of only 18%, P fertiliser main effect. Increasing the application rate of SS increased S uptake by 60% while ES only resulted in a 26% increase in S uptake (rate by S source interaction).
Application of SS resulted in a 10% lower Mo content (mg Mo/kg canola biomass) than when ES was applied, S source main effect (Figure 1d). Maximum Mo uptake by canola occurred at an SS rate of 10 kg S/ha which was 19% higher than the control, but the application of an additional 5 kg S/ha to 15 kg S/ha resulted in a 25% reduction in Mo uptake by canola.
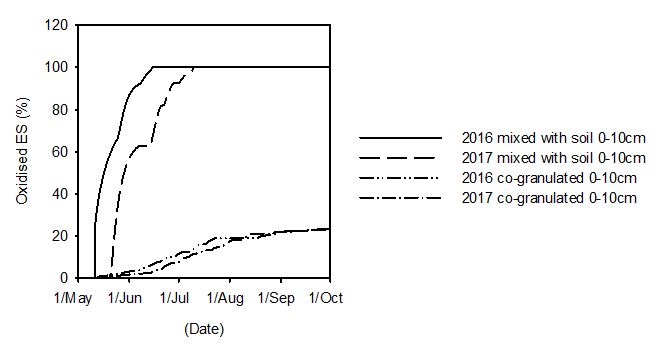
The effect of seasonal conditions on the rate of ES oxidation was examined by comparing predicted ES oxidation using the 2016 and 2017 rainfall data (Figure 2). These two years have contrasting rainfall during June. In 2016, there was 70 mm distributed evenly during June compared to the low rainfall of 37 mm in 2017 of which 14 mm occurred on 31 June. Under these seasonal conditions, the McCaskill and Blair (1989) ES oxidation model predicted 15 kg ES/ha would be oxidised by 16 June in 2016 while in 2017 this amount was not oxidised until the 9 July. Predicted co-granulated ES oxidation is much less than soil incorporated ES oxidation with a smaller effected of the seasonal conditions on the rate of ES oxidation (Figure 2).
Figure 2. Predicted ES oxidation rates of 15 kg S/ha when mixed with the soil or co-granulated applied on 6 May 2016, and 2107 using McCaskill and Blair (1989) and Degryse et al. (2016) models respectively linked link into a water balance model (Anderson et al. 2011).
Conclusion
Elemental sulphur coated phosphorus fertiliser did not meet the S requirements of canola as illustrated by phosphorus fertiliser coated ES having a 16% lower grain yield compared to applying additional SS fertiliser. Elemental sulphur is a slow release fertiliser with its release rate dependent on the presence of S-oxidising bacteria Acidithiobacillus population within the soil, the amount of soil exposed surface area of the ES, the environmental condition of soil water and temperature and soil properties pH and carbon content (Zhao et al. 2017). In this experiment, low rates of ES oxidation, determined by canola grain yield response to increasing ES application, was low due to the soil contained low levels of S-oxidising bacteria (2.25 - 3.66 x106 per gram soil) and dry seasonal conditions, especially during May and June 2017. Perhaps fertiliser granules could be inoculated with S-oxidising bacteria to increase ES oxidation rates (Mattiello et al. 2017). Higher ES oxidation rates have been observed by Degryse et al. (2016b) who observed that, under glasshouse conditions or ideal soil water conditions, 50-60% of ES contained in GranulockSS® was oxidised within 120 days but this rate of oxidiation resulted in lower S uptake compared to SS treatment.
Application of SS of more than 10 kg S/ha reduced Mo uptake resulting in a 14-24% reduction in canola biomass production. Mo and sulphate behave similarly and have similar structure and are taken up by the same root transporters (Zimmer and Mendel 1999). Hence, reduced uptake of Mo at sulphate rates of greater than 10 kg S/ha most likely occurred due to competition for absorption at the root transporter sites.
Canola has a higher S requirement than cereals and legumes (Anderson et al. 2006). Hence, smaller increases in grain yield would have expected for wheat and lupin. In this experiment, the soil test in the 0-30 cm was 3.2 mg S/kg which was predicted to provide a 67% response to S fertiliser application (Anderson et al. 2013). A lower response of 46% was observed in this experiment. The lower response to sulphate application could be due to reduced Mo uptake at the high sulphate rates and dry seasonal conditions.
Additional research should be done looking at the effectiveness of ES coated P fertiliser in wetter seasonal conditions and on soils which have higher populations of S-oxidising bacteria or including S-oxidising bacteria in the ES coated fertilisers when the soils are expected to have low levels of S-oxidising bacteria.
Further information Geoff Anderson 08 96902104 or geoff.anderson@dpird.wa.gov.au
Acknowledgements
Project number FFPJ04 ‘Boosting Grains Research and Development project’, funded by the WA State Government. Professor Graeme Blair (UNE) provided advice on procedures for coating ES onto fertiliser granules, and this is greatly appreciated.
References
Anderson, G., Bell, R., Brennan, R. and Chen, W. (2011) Soil management calculator for predicting phosphorus losses under cropping systems in Western Australia. In: 2011 WA Agribusiness Crop Updates, 23 - 24 February, Perth, Western Australia
Anderson GC, Fillery IRP, Ripper FH, Leach BJ (2006) Sulfur mineralisation in a coarse-textured soil after different sulfate fertilisation histories, and yield responses of wheat and lupin. Australian Journal of Soil Science 44, 165–174.
Anderson G, Peverill K, Brennan R (2013) Soil sulphur – crop response calibration relationships and criteria for field crops grown in Australia. Crop & Pasture Science 64, 523–530.
Barrow NJ (1971) Slowly available sulphur fertilizers in south-western Australia 1. Elemental sulphur. Australian Journal Experimental Agriculture and Animal Husbandry 11, 211-216.
Degryse F, Rodrigo C. da Silva RC, Baird R (2016a) Effect of co-granulation on oxidation of ES: theoretical model and experimental validation. Soil Science Society America Journal 80, 1244–1253.
Degryse F, Rodrigo C. da Silva RC, Baird R (2016b) Availability of fertiliser sulphate and elemental sulphur to canola in two consecutive crops. Plant & Soil 398, 313–325.
Pitala J, Blair G (2017) Sulfur availability from elemental S coated fertilisers. Proceedings of the 18th Australian Society of Agronomy Conference, 24 – 28 September 2017, Ballarat, Australia. Australian Society of Agronomy
McCaskill MR, Blair GJ (1989) A model for the release of sulfur from elemental S and superphosphate. Fertilizers Research 19, 77-84.
Zhao C, VVSR Gupta, Degryse F, McLaughlin MJ (2017) Abundance and diversity of sulphur-oxidising bacteria and their role in oxidising elemental sulphur in cropping soils. Biology and Fertility of Soils 53, 159–169.
Zimmer W, Mendel R (1999) Molybdenum metabolism in plants. Plant Biology 1, 160–168.
Mattiello EM, da Silva RC, Degryse F, Roslyn Baird R, Gupta VVSR, McLaughlin MJ (2017) Sulfur and zinc availability from co-granulated Zn-enriched elemental sulfur fertilizers. Journal of Agricultural and Food Chemistry 65, 1108−1115.
Was this page helpful?
YOUR FEEDBACK