Wheat powdery mildew epidemiology and crop management options
Author: Jason Bradley and Geoff Thomas, DPIRD | Date: 25 Feb 2019
Key Messages
- Wheat powdery mildew (WPM) infection is favoured by temperatures of 15-23oC and is sensitive to periods over 25oC. Rate of infection and spore production are optimum at 20-23oC. Parameters favouring WPM can be used to identify the periods of increased epidemic risk and guide the need formanagement intervention in the different climatic regions of the WA wheatbelt.
- Fertiliser applications can influence powdery mildew severity. With increasing nitrogen fertilisation, plants are effectively more susceptible to WPM and crop canopies are more dense favouring epidemic development. These crops will require more intensive monitoring and disease management. While responses were small and inconsistent, experiments suggest that maintenance of adequate potassium nutrition can benefit in helping reduce powdery mildew development.
- All commercial wheat varieties tested were susceptible as seedlings to all powdery mildew isolates from across the wheatbelt. Some variability in virulence of isolates was evident on alternate resistance genes, however until varieties with improved resistance are released placing selection pressure on the powdery mildew population, variety responses should be consistent across the wheatbelt and reflect NVT disease ratings.
Aims
Wheat powdery mildew caused by the fungus Blumeria graminis f.Sp tritici is a significant pathogen in wheat growing areas with temperate maritime climates worldwide. In Australia it can cause yield losses of up to 25%. The disease is favoured by stubble retention, high seeding and nitrogen fertiliser rates and large areas planted with susceptible varieties.
The disease is sensitive to environmental conditions and can vary from season to season. Control can be complicated due to the multiple, rapid life cycles (5-12 days) and wide dispersal of large numbers of small, light, wind-borne spores. Understanding the diversity of powdery mildew pathotypes (virulence frequencies) in WA is important in the development of wheat lines with effective, enduring resistance and for management of existing commercial varieties.
Nitrogen and potassium application has been shown to influence disease severity. How these nutrients influence a crop’s susceptibility to WPM will influence integrated disease management (IDM) practices.
The main aims of this research are to i) investigate the diversity of virulence in WPM isolates from across WA, ii) measure the influence of growth stage and temperature on infection efficiency, latent period and severity of WPM, iii) determine the impact of macro-nutrients nitrogen and potassium on WPM infection and iv) understand how varietal resistance influences response to these factors.
Method
WPM virulence spectrum
Wheat powdery mildew samples were collected from 31 locations across the WA wheatbelt in 2016 and 2017. Each sample was stored and bulked up in isolation to maintain purity. All samples were screened in a glasshouse against a differential set of wheat lines with known resistance genes, (Golzar et al., 2016) and a set of commercial varieties with varying reported resistance levels to WPM. Inoculum was dusted onto plants grown in pots at 2 leaf stage (31 samples) and head emergence (5 samples) and disease assessed after 12 days using a 0-5 scale where 0 = no infection and 5 = severe sporulation.
Temperature thresholds (latent period, sporulation, spore germination)
All temperature threshold experiments were conducted using the variety Wyalkatchem which is rated susceptible very susceptible (SVS) to WPM. Under laboratory conditions and in a controlled environment, excised leaf fragments (3 replicate plates of 8 leaf fragments) and 3 leaf seedlings (2 replicate pots of 5 seedlings) were dusted with spores of a WPM isolate collected from DPIRD Woorree research station in 2016. They were then held under plastic covers to maintain humidity and infection from a single isolate at constant temperature for a period of two weeks. Temperatures tested were 5, 10, 15, 20, 23.5, 25ºC. Each temperature experiment was paired with baseline control plants inoculated at the same time and kept at 20ºC. Daily observations were carried out for germination of inoculated spores, lesion development, presence of sporulation and disease severity. Spore production in lesions was counted 12 days after inoculation.
Using the same experimental design, inoculated leaf fragments and seedlings were exposed to 25ºC for 6, 12, 24, and 30 hours post inoculation before being returned to the “optimum” temperature of 20ºC and evaluated for infection type and percentage leaf area infected at 4, 6, 7 and 11 days after inoculation.
Growth stage impact on disease severity
In controlled environment conditions (average 17oC), three varieties (Scepter SVS, Mace MSS, Magenta MR) were sown in pots at three times to achieve three growth stages (~Z13, Z32, Z43). All plants were inoculated with a WPM isolate from DPIRD Woorree research station on the same day. Plants were assessed for leaf area affected by mildew on the top three fully expanded leaves 14 days after inoculation.
Nitrogen and potassium nutrition
Field trials to examine impact of nitrogen and potassium nutrition on WPM development and severity were carried out as individual experiments at DPIRD Medina Research station in 2017 (Scepter, Zen, Mace, Tungsten, Magenta) and UWA Shenton Park field station in 2018 (Scepter, Mace, Magenta). Trials were sown in small plots as a split plot design with 4 replicates, with nutrition as main-plots and variety as sub-plots.
Baseline soil nutrient concentrations were assessed from 0-10cm, 10-30cm and 30-60cm soil samples submitted to CSBP laboratories for analysis before planting. Fertiliser applications for both years are listed in table 3. Nitrogen was added as urea, and potassium as muriate of potash applied at up to three times (0, 3 and 6 weeks after sowing).
Powdery mildew was introduced by natural spread from inoculated susceptible spreader rows adjacent to each plot. Disease severity was measured as leaf area affected by WPM in 5 replicate plants per plot on a fortnightly basis from Z39 (flag leaf emergence) in each plot.
Table 1. Nitrogen and potassium fertiliser applied in field and glasshouse trials in 2017 and 2018
Field trials* | Glasshouse trials# | ||||||||
|---|---|---|---|---|---|---|---|---|---|
Nitrogen (kg /ha) | Potassium (kg /ha) | Nitrogen (kg /ha) | Potassium (kg /ha) | ||||||
Nutrient Level | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | |
Nil | - | - | - | - | 0 | 0 | 0 | 0 | |
Inadequate | 40 | 40 | 20 | 0 | 40 | 20 | 20 | 5 | |
Low | 80 | 60 | 40 | 10 | 80 | 40 | 40 | 10 | |
Adequate | 120 | 100 | 80 | 20 | 120 | 80 | 60 | 15 | |
Luxury | 160 | 180 | 160 | 40 | 160 | 120 | 80 | 20 | |
*baseline levels in field sites (kg/ha): 18 K, 2 N (2017) and 32 K, 1 N (2018)
#baseline levels in coarse river sand used in glasshouse trials (kg/ha): 17 K, 1 N (2017) and 19 K, 0 N (2018)
Glasshouse experiments in 2017 and 2018 were carried out under controlled environment conditions using similar varieties to field experiments. Plants were sown into coarse river sand (baseline nutrient concentration assessed by CSBP laboratories) with nitrogen and potassium as liquid formulation applied at up to 3 times to achieve application rate. Plants were inoculated by dusting with WPM spores at Z13 (3-4 leaf stage) and disease severity assessed as leaf area on top 3 leaves affected by WPM at stem elongation (~Z31-32) and full head emergence (~Z59-65), 21 and 42 days after inoculation.
Statistical analysis
Analysis of variance (Genstat 19th edition) were performed on data from field and glasshouse trials. Some data required square root transformation to stabilise variance (van Burgel, pers. comm.)
Results
PM virulence
Thirty one WPM isolates sourced from widespread locations in five Port zones in 2016 and 2017 were assessed for virulence (Geraldton 4, Metro 2, Kwinana 7, Albany 11, Esperance 7). All isolates were virulent on seedlings of commercial lines tested (Arrino, Binnu, Calingiri, Corack, Mace, Magenta, Wyalkatchem and Yitpi), adult plant responses were evident in Binnu, Magenta, and Yitpi, reflecting NVT rankings for these varieties. Screening at seedling stage showed across the board susceptibility to isolates in four differential lines (incl. genes Pm1a, Pm5a, Pm8, Pm17), resistance to all isolates in 11 differential lines (incl. genes Pm2, Pm3a, Pm3c, Pm3e, Pm4a, Pm13) and varying response in five lines (incl. genes Pm6, Pm7, Pm24, Pm28). Virulence on Pm24 was only detected in 2017 and isolates from Metro and Esperance port zones did not have virulence on Pm7.
Temperature thresholds (incubation period, latent period, sporulation, spore germination)
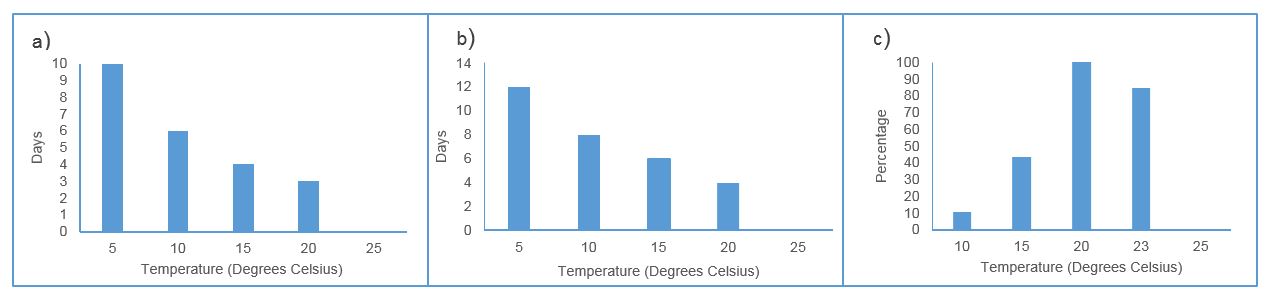
The incubation period (time to appearance of first symptoms) and latent period (time to start of sporulation) of wheat powdery mildew more than doubled at 5 ºC (~12 days) compared to 20ºC (~4 days). Spore production in lesions was also delayed and reduced at lower temperatures (Figure 1 a, b, c). Linear regression of data showed a significant relationship between temperature and % spore production (ANOVA groups: 20-a, 23.5-ab, 15-bc, 10-c). In additional experiments comparing 23.5ºC to 20 ºC (data not shown) latent period, disease severity and spore germination rates were similar to 20 ºC.
Figure 1: Impact of incubation at a range of constant ambient temperatures on a) incubation period, b) latent period and c) comparative spore production at 12 days after incubation of Wyalkatchem seedlings or excised leaves.
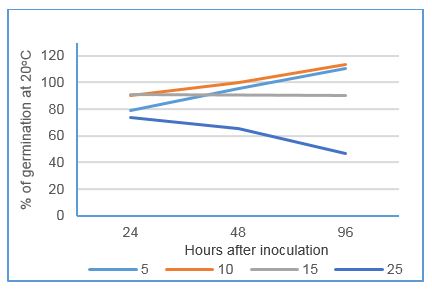
Figure 2: Impact of incubation at a range of constant ambient temperatures on germination of wheat powdery mildew spores (expressed as a percentage of that at 20 degrees)
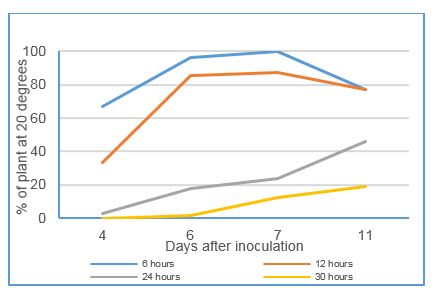
Figure 3: Impact on WPM infection over 11 days after 4 different exposure times to 25 degrees, expressed as a percentage of that at 20 degrees (LSD at 4d 8.6%, 6d 18.1%, 7d 29.4%, 11d 19.6%)
Approximately half of spores counted germinated when held at 20ºC. When compared to the 20ºC control, spores incubated at 5, 10 and 15ºC showed similar germination rates, ranging from 80-110% of the control over the 96 hour test. However, viability of spores held at 25ºC diminished compared to the 20ºC control (Figure 2).
Exposure to 25ºC for periods of time immediately post inoculation (prior to returning to 20 ºC) progressively delayed and reduced severity of infection on seedlings and leaf fragments compared to the 20ºC control (Figure 3). Six and 12 hours exposure reduced severity by 15% while 24 and 30 hours exposure significantly reduced severity at all times of assessment compared to the control.
Plant growth stage
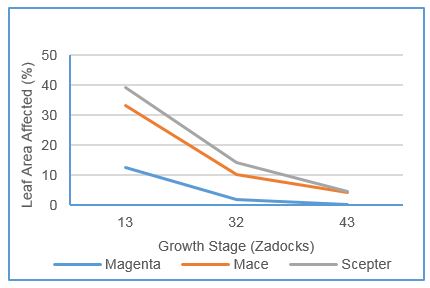
Susceptible (Scepter) and moderately susceptible to susceptible (Mace) plants inoculated at Z13 and Z31 had significantly greater severity of disease compared to those inoculated at Z43. Moderately resistant Magenta exhibited minor levels of disease at seedling stage but had significantly less disease than Scepter or Mace at all growth stages (Figure 4).
Figure 4: Wheat powdery mildew severity, assessed 5 weeks after inoculation, on three varieties inoculated at three growth stages. Scepter rated SVS, Mace MSS, and Magenta MR to powdery mildew (LSD: GS = 3.27%, variety = 4.34%, var x GS = 6.64% p=0.05)
Nitrogen
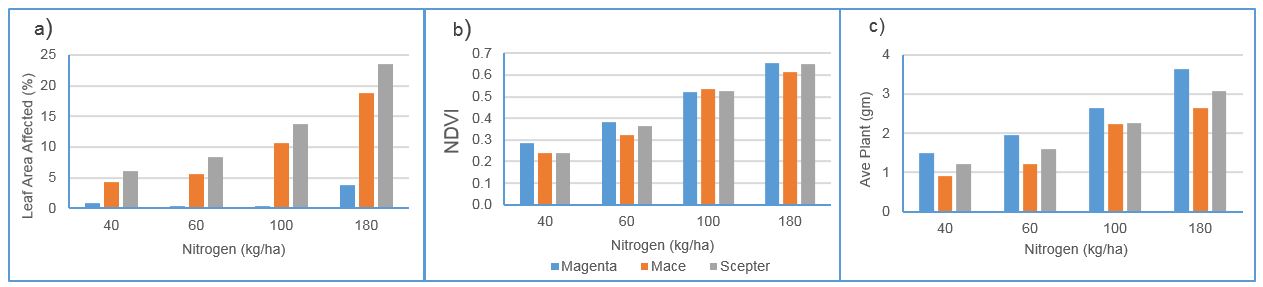
In three out of four experiments, WPM severity was greater with higher rates of applied nitrogen fertiliser. Variations in starting soil nitrogen concentration and seasonal conditions meant that establishing a consistent threshold application level was not possible. At Medina (2017) severity of wheat powdery mildew in susceptible varieties Scepter and Mace increased with increasing application rate up to the ‘luxury’ rate (120 kg/ha). Disease severity in Magenta (MRMS) did not respond to nitrogen rate (data not shown). Similarly at Shenton Park in 2018, increasing nitrogen application resulted in greater WPM severity in the susceptible varieties but not significantly so in Magenta, despite growth in all varieties being nitrogen responsive, having increased NDVI readings and yield at increasing rates (Figure 5 a,b,c).
Figure 5: Impact of rates of nitrogen applied on a) powdery mildew severity (LSD: N level 2.1%, variety 1.7%, N x var 3.3%), b) NDVI (LSD: N level 0.03, variety 0.02, N x var 0.04) and c) yield of three wheat varieties at Shenton Park in 2018 (LSD: N level 0.31g, variety 0.26g, N x var 0.5g)
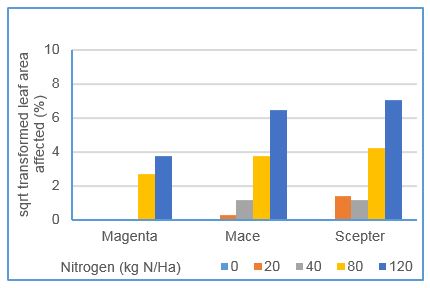
No response was evident in the 2017 glasshouse experiment, however significant differences in disease severity were evident between nitrogen treatments in the 2018 glasshouse experiment.
Replicating the field responses, disease responses in Magenta were minor, however in the susceptible varieties Mace and Scepter, significant differences were evident between nitrogen rates. At inadequate and low rates, plants suffered nitrogen deficiency symptoms and disease levels were negligible. At adequate and luxury levels, plants were healthy and disease severity showed a significant increasing trend (Figure 6). With glasshouse trial design eliminating canopy impacts of higher nitrogen rates, this result indicates nitrogen was directly influencing plant disease response.
Figure 6. Impact of rates of nitrogen applied on powdery mildew severity, of three wheat varieties grown in glasshouse conditions in 2018. Scepter rated SVS, Mace MSS, and Magenta MR to powdery mildew. Data shown has undergone a square root transformation (LSD: variety 0.48%, N level 0.95%, N x var 1.3%)
Potassium
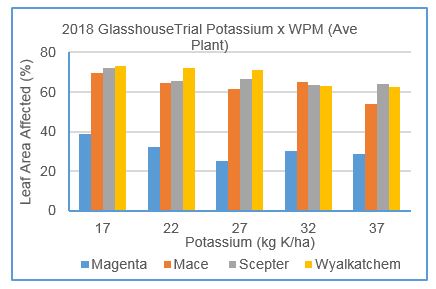
Significant impact of application rates of muriate of potash on wheat powdery mildew were evident at only two of four experiments (Medina 2017 and Glasshouse 2018). Baseline soil potassium concentrations in trials were only marginally below recommended thresholds and establishment of potassium deficiency was problematic. Response to potassium is inverse of nitrogen response, greater disease severity occurs when potassium is deficient.
In 2017, moderately severe WPM was present in all plots, however disease severity in three of five varieties (Magenta, Tungsten, Zen) was marginally higher (10-15%) at inadequate potassium concentration (of 20 and 40kg applied) compared to adequate (80 and 160kg applied).
Similarly, in the 2018 glasshouse experiment, under high disease pressure, two varieties (Mace and Wyalkatchem) showed significant reduction of disease severity (10-15%) between minimum and maximum fertiliser treatments (Figure7).
Figure 7: Impact of rates of potassium applied on powdery mildew severity, of four wheat varieties grown in glasshouse conditions in 2018. Magenta rated MR, Mace MSS, Scepter and Wyalkatchem SVS to powdery mildew (LSD: variety 4.7%, K level 8.9%, K x var 12.4%)
Conclusion
Understanding the environmental, genetic and agronomic factors which influence wheat powdery mildew development are important for developing crop monitoring and disease management programs across the climatic zones of the WA wheatbelt.
The response of commercial varieties tested reflected current NVT ratings, with all being susceptible as seedlings and a small number showing, at best, a moderately resistant to moderately susceptible (MRMS) response as adult plants. The dominance of susceptible varieties (e.g. Mace) has placed very little selection pressure on the WPM population in WA and correspondingly only a small proportion of tested lines (resistance genes) showed differential responses between isolates. Until new seedling resistance genes are widely deployed placing greater selection pressure on the population, variety responses should remain constant across the wheatbelt and management should reflect the NVT ratings.
These experiments have shown that factors such as optimum nitrogen nutrition and disease onset at early growth stages magnify risk of powdery mildew in susceptible varieties, effectively rendering plants more susceptible. The use of seed dressings and in-furrow fungicides has been shown to effectively delay powdery mildew onset (Thomas et al. 2017) and may be of extra benefit where early onset of disease at more susceptible growth stages is expected (e.g. green bridge years), or when higher rates of nitrogen are applied at early growth stages. At later growth stages, high nitrogen status can result in greater susceptibility of plants and denser canopies, increasing disease severity and potentially placing greater pressure on fungicide efficacy.
Understanding environmental drivers of disease risk can provide guidance on how disease may develop in different regions of WA and potentially how fungicide programs might be tailored to suit how epidemics develop in these regions. In a typical year, with inoculum present, northern growing regions are at risk of experiencing the earliest outbreaks of WPM in the WA wheatbelt. For Geraldton, peak rainfall is in June-July, with optimum daily conditions averaging 20-21oC and nights averaging 10-11oC, an ideal environment for a rapid lifecycle of WPM. Epidemics will spread quickly in susceptible varieties in June - August, however by September rainfall decreases (lowering canopy humidity) and the number of days over 25oC increases markedly (e.g.14 days in 2015) reducing likelihood of successful WPM infection and disease spread. In contrast, regions south of Perth, like Katanning, are likely to have epidemics developing later in the season. Winter is significantly cooler (av. day temp 15oC and night 5-6oC) resulting in a longer latent period and decreased sporulation but more suitable temperatures persist longer into spring with rainfall maintained, and fewer days over 25oC. The Esperance region has seen significant disease outbreaks in several years, early disease development is favoured by mild April and May conditions with few days over 25 degrees. Peak rain is July-August with optimum temperatures (including few days over 25oC) continuing into October. This environment appears to have the greatest span of favourable weather conditions for the disease with mildew developing rapidly in spring and affecting both foliage and heads of susceptible varieties. This has been reflected in epidemic development observed in recent seasons.
References
Golzar, H., Shankar, M., and D’Antuono, M. (2016) Responses of commercial wheat varieties and differential lines to western Australian powdery mildew (Blumeria graminis f. Sp. tritici) populations. Australasian Plant Pathology 45:347-355.
Thomas, G., Beard, C., Jayasena, K., Hills, A., Bradley, J., and Smith, A., (2017) Fungicides at seeding for management of cereal foliar diseases: powdery mildew in wheat GRDC Grains Research Update, Perth
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
Acknowledgments
DPIRD research support units at Medina (G D’Adhemar), Esperance (C Matthews), Geraldton (S Cosh, A Smith) and Katanning (D.Cox). D. Jorgensen and R.O’Leary (DPIRD)
DPIRD Biometrician, Andrew van Burgel
DPIRD and Royalties for Regions funding (FFPjP09)
Paper reviewed by Ciara Beard, DPIRD
Was this page helpful?
YOUR FEEDBACK