Implications of continuing dry conditions on cereal disease management
Author: Steven Simpfendorfer (NSW DPI) | Date: 26 Feb 2020
Take home messages
- Due to a combination of factors there is likely to be increased cereal plantings in 2020, once the opportunity arises
- Failed pastures with decent levels of grass development are potentially high risk scenarios for cereal diseases in 2020 as grasses host many of the causal pathogens
- Unfortunately, prolonged dry conditions increase the risk of cereal diseases including Fusarium crown rot and rhizoctonia root rot
- However, steps can be taken to minimise impacts which include:
1. Know before you sow (e.g. PREDICTA®B)
2. Implementing pre-sowing management options
3. Sowing quality seed known to have both good germination and vigour
4. Assessing root health and infection levels around heading – you need to ‘dig deeper’ than just leaf diseases!
Introduction
Unfortunately, much of central NSW experienced a relatively dry winter cropping season again in 2019. These conditions, especially with hotter and drier conditions during grain filling, are ideal for the expression of Fusarium crown rot as whiteheads and resulting yield loss. Fusarium crown rot, caused predominantly by the stubble-borne fungus Fusarium pseudograminearum, infects all winter cereal crops (wheat, barley, durum, triticale and oats) and numerous grass weed species also host this pathogen. However, a key point is that dry conditions do not just have implications for Fusarium crown rot management. There are other potential cereal disease implications that need to be considered by growers and management strategies implemented to maximise profitability when recovering from drought.
Extended dry conditions in 2018 and 2019, possibly longer in some areas, has a range of potential implications on farming systems which can include:
- Reduce stubble cover – increasing wind erosion, reducing fallow efficiency and limiting stored soil moisture levels
- Reduced decomposition of crop residues which can extend inoculum survival to 2 to 4+ years
- Reduced animal stock numbers – extended dry has seen sheep and cattle numbers decline which will take a number of seasons to recover
- Reduced survival of pastures in mixed cropping systems
- Later seasonal breaks reducing opportunities for canola establishment in some districts
- Widespread baling of cereal crops for hay in 2018 and 2019
- Increased pressure on available planting seed for establishing crops in 2020.
Although many of these issues are common across continuous and mixed cropping enterprising, as a general rule those operations that have opted for more intensive broadacre crop production are hopefully more aware of potential pitfalls around limiting cereal diseases and ensuring quality of planting seed. The lack of animal stock, failure of pastures and need for ground cover is likely to see a substantial increase in the area of cereals planted, especially in mixed farming systems once the drought breaks. Grass species and grass weeds tend to dominate as legume species decline in pasture mixes over time and with moisture stress. These are therefore potentially higher risk paddocks for cereal diseases as the grasses serve as alternate hosts for pathogens such as Fusarium pseudograminearum (Fusarium crown rot), Bipolaris sorokiniana (common root rot), Rhizoctonia solani (rhizoctonia root rot), Gaeumannomyces graminis var. tritici (take-all), root lesion nematodes and some leaf diseases (e.g. barley grass hosts net-blotch pathogen Pyrenophora teres).
When the drought does break in impacted regions, hopefully in 2020, growers will be driven by two key factors. The first will be to generate cash flow and the second will be to restore groundcover to bare paddocks through the planting of winter cereals. This will potentially occur with little regard to the risk posed by plant pathogens and the quality of available planting seed. Maximising the profitability of crop production is going to be critical to many farming operations once the drought breaks. The following paper highlights some of the potential issues for consideration by growers and agronomists from a cereal pathology view point. Some practical steps that can be taken to hopefully minimise losses are also outlined.
Step 1: Know before you sow
Although paddock history can be a good guide to potential disease issues, extended dry conditions can allow damaging inoculum levels to still persist from 2-4+ seasons ago. Hence, growers need to consider the longer-term sequences within paddocks. How cereal stubble was handled over prolonged dry conditions can also influence the survival and distribution of cereal pathogens. Paddock history is only a guide and provides no quantitative information on the actual level of risk posed by different cereal diseases.
Consider testing paddocks using PREDICTA®B. This would be especially useful for paddocks coming out of failed pastures which may have become dominated by grasses. PREDICTA®B is a quantitative DNA based soil test which provides relative risk or population levels for a wide range of pathogens that can be used to guide management decisions. However, ensure you are using the latest recommended PREDICTA®B sampling strategy which includes the addition of cereal stubble to soil samples (see useful resources). Addition of cereal stubble (or grass weed residues if present in pasture paddocks) improves detection of stubble-borne pathogens which cause diseases such as Fusarium crown rot, yellow spot in wheat and net-blotches in barley. Considerable GRDC co-funded research has been conducted nationally over the last 5 years to improve the recommended sampling strategy, refine risk categories and include additional pathogens or beneficial fungi (AMF) on testing panels. Recent paddock surveys have highlighted that a single pathogen rarely exists in isolation within individual paddocks but rather multiple pathogens occur in various combinations and at different levels. PREDICTA®B is world leading technology that can quantitatively measure these pathogen combinations within a single soil + stubble sample. Given extended dry conditions the two key cereal diseases of concern for 2020 in central NSW are likely to be Fusarium crown rot and rhizoctonia root rot. The risk of both of these diseases can be determined by PREDICTA®B.
Alternately, cereal stubble or grass weed residues can be collected from paddocks and submitted to NSW DPI laboratories in Tamworth as a ‘no charge’ diagnostic sample (see contact details). Samples are plated for recovery of only two pathogens which cause Fusarium crown rot or common root rot and provide no indication of other potential disease risks.
Step 2: Consider pre-sowing management options
Generic management options are provided with PREDICTA®B test results which are tailored to the actual levels of different key pathogens detected within a sample. Your PREDICTA®B accredited agronomist should also be able to assist with interpretation which can be daunting given the number of pathogens covered by the testing. NSWDPI are also happy to discuss results (PREDICTA®B or stubble testing) and work through potential management options (see contact details).
Assuming main concern is Fusarium crown rot. Based on the following PREDICTA®B or stubble test results pre-sowing management options include:
Below detection limit (BDL) or low
No restrictions, ensure good crop agronomy
Medium
Consider sowing a non-host pulse or oilseed crop with good grass weed control.
If sowing cereal then:
- Avoid susceptible wheat or barley varieties, durum is higher risk but oats are fine
- Sow at the start of a varieties recommended window for your region
- Consider inter-row sowing (if previous cereal rows are still intact) to limit contact with inoculum
- Do not cultivate - it will spread inoculum more evenly across paddock and into infection zones below ground
- Be conservative on nitrogen application at sowing (Figure 1) as this can exacerbate infection (e.g. consider split application) but ensure a maintenance level of zinc is applied
- Be aware that current seed treatments registered for Fusarium crown rot suppression provide limited control
- Determine infection levels around heading (see step 4).
High
Consider sowing a non-host pulse or oilseed crop with good grass weed control.
If sowing cereal then:
- Choose a more tolerant wheat or barley variety for your region to maximise yield and profit (Table 1), durum is very high risk with yield loss >50% probable in a tough finish but oats are still a decent option
- Sow at the start of a varieties recommended window for your region as this can half the extent of yield loss
- If a late break occurs consider switching to a quicker maturing wheat variety or go with barley to limit exposure to heat stress during grain filling which exacerbates yield loss
- Consider inter-row sowing (if previous cereal rows are still intact) to limit contact with inoculum
- Do not cultivate - it will spread inoculum more evenly across paddock and into infection zones below ground
- Be conservative on nitrogen application at sowing (Figure 1) as this can exacerbate infection (e.g. consider split application) but ensure a maintenance level of zinc is applied
- Be aware that current seed treatments registered for Fusarium crown rot suppression provide limited control and get to a Syngenta learning centre in 2020
- Determine infection levels around heading (see step 4) and be prepared from sowing to cut for hay or silage if required.
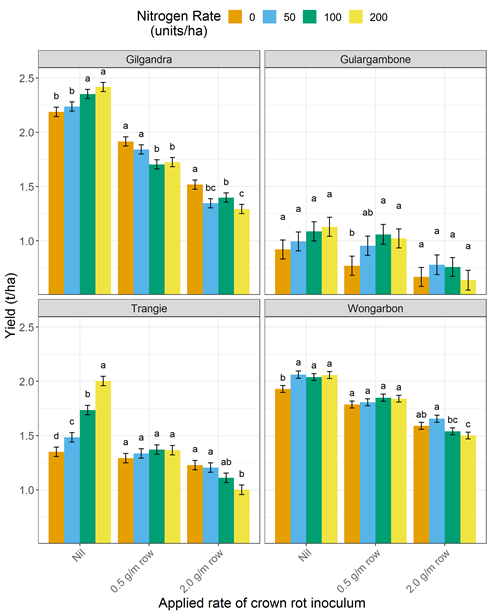 Figure 1. Interaction of nitrogen nutrition and crown rot infection on bread wheat (Suntop and EGA Gregory) yield across four sites in central NSW in 2018.
Figure 1. Interaction of nitrogen nutrition and crown rot infection on bread wheat (Suntop and EGA Gregory) yield across four sites in central NSW in 2018.
Note: Nil applied inoculum represents a BDL/low risk, 0.5 g/m row a medium risk and 2.0 g/m row a high risk of crown rot infection.
Table 1. Average yield (t/ha), yield loss from crown rot (%), screenings (%) and lost income from crown rot ($/ha) of four barley, 5 durum and 20 bread wheat entries in the absence (no added CR) and presence (added CR) of crown rot inoculum averaged across 50 sites in central/northern NSW and southern Qld – 2013 to 2017.
Varieties within crop species ordered from highest to lowest yield in added CR treatment. Lost income and income in added CR treatment based solely on reduced yield (t/ha) in added CR treatment or absolute yield (t/ha) in this treatment multiplied by average grain price of $220/t for barley, $240 for AH and $300/t for APH bread wheat and $350/t for durum. Grain quality impacts and variable costs including PBR not considered.
Crop | Variety | Quality | Yield (t/ha) | Yield loss | Screenings (%) | Lost income from crown rot | Income added CR | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
| No added CR | Added CR | (%) | No added CR | Added CR | ($/ha) | ($/ha) |
Barley | La Trobe | 4.17 | 3.59 | 14.0 | 6.5 | 8.4 | 128 | 790 | |
| Spartacus | 4.18 | 3.58 | 14.3 | 2.9 | 4.6 | 131 | 788 | |
| Commander | 4.09 | 3.40 | 16.8 | 6.1 | 8.2 | 151 | 748 | |
| Compass | 4.20 | 3.39 | 19.4 | 2.1 | 2.9 | 179 | 745 | |
Durum | Lillaroi | 3.79 | 3.00 | 20.8 | 3.2 | 5.9 | 275 | 1050 | |
| Bindaroi | 3.88 | 2.92 | 24.7 | 2.7 | 5.8 | 336 | 1023 | |
| Jandaroi | 3.48 | 2.64 | 24.3 | 4.1 | 9.2 | 296 | 923 | |
| Caparoi | 3.34 | 2.20 | 34.1 | 9.0 | 16.5 | 399 | 770 | |
| AGD043 | 2.72 | 1.65 | 39.1 | 3.8 | 13.8 | 372 | 579 | |
Bread | Beckom | AH | 4.57 | 3.94 | 13.9 | 8.8 | 12.7 | 153 | 944 |
Mustang | APH | 4.17 | 3.67 | 11.9 | 5.2 | 7.0 | 148 | 1102 | |
| Mitch | AH | 4.08 | 3.51 | 13.9 | 7.7 | 10.2 | 136 | 842 |
| Reliant | APH | 4.18 | 3.50 | 16.3 | 5.3 | 8.1 | 204 | 1051 |
| Suntop | APH | 3.99 | 3.46 | 13.3 | 7.3 | 9.6 | 160 | 1037 |
| Sunguard | AH | 3.81 | 3.35 | 12.0 | 6.2 | 8.7 | 110 | 804 |
| Spitfire | APH | 3.86 | 3.34 | 13.3 | 5.8 | 8.0 | 154 | 1003 |
| Gauntlet | APH | 3.92 | 3.29 | 16.1 | 4.4 | 7.0 | 189 | 987 |
| Lancer | APH | 3.88 | 3.27 | 15.8 | 4.8 | 7.1 | 184 | 981 |
| Sunmate | APH | 4.02 | 3.23 | 19.6 | 6.4 | 9.7 | 237 | 969 |
| Coolah | APH | 4.03 | 3.21 | 20.4 | 5.8 | 9.4 | 247 | 962 |
| Flanker | APH | 4.04 | 3.12 | 22.8 | 6.0 | 10.4 | 277 | 936 |
| Dart | APH | 3.73 | 2.99 | 19.9 | 9.3 | 12.8 | 223 | 897 |
| EGA Gregory | APH | 3.90 | 2.89 | 25.9 | 6.7 | 11.4 | 303 | 868 |
| Viking | APH | 3.48 | 2.89 | 17.1 | 10.9 | 16.8 | 179 | 866 |
| Lincoln | AH | 3.88 | 2.78 | 28.3 | 8.6 | 12.8 | 264 | 668 |
| Crusader | APH | 3.43 | 2.76 | 19.4 | 8.3 | 13.4 | 199 | 829 |
| QT15064R | APH | 3.68 | 2.73 | 25.7 | 8.3 | 15.1 | 284 | 819 |
| Suntime | APH | 3.43 | 2.62 | 23.6 | 10.6 | 17.2 | 243 | 787 |
| Strzelecki | AH | 3.03 | 2.17 | 28.3 | 12.0 | 18.0 | 206 | 521 |
|
| Lsd (P=0.05) | max. 0.137 | max. 1.37 | |||||
Note: The extent of yield loss associated with crown rot infection varied between seasons and sites being 21% in 2013 (range 13% to 55% across nine sites), 22% in 2014 (range 6% to 47% across 12 sites), 18% in 2015 (range 7% to 42% across 12 sites), 13% in 2016 (range 6% to 29% across 11 sites) and 29% in 2017 (range 20% to 45% across six sites) averaged across varieties.
Assuming the main concern is rhizoctonia root rot (AG8), which is particularly favoured in lighter red soils. Based on the following PREDICTA®B test results pre-sowing management options include:
Below detection or low
No restrictions, ensure good crop agronomy.
Medium or high
Consider sowing a non-host pulse or oilseed crop with good grass weed control.
If sowing a cereal then:
- Avoid pre-sowing sulfonylurea herbicides which can restrict early root growth which exacerbates infection
- Consider slightly increasing sowing rate to compensate for potential tiller losses
- Plant at the start of a varieties recommended window for your region as more rapid root growth in warmer soil allows the primary root system to escape significant infection
- Sow wheat instead of barley as it is less susceptible to rhizoctonia, oats are also a good option
- Soil disturbance below the seed (ideally 5-10 cm) at sowing promotes rapid root growth away from rhizoctonia and disrupts the hyphal network, risk is greater with single disc seeders than knife points
- Ensure good nitrogen and phosphorus nutrition as deficient crops are more susceptible
- Current seed treatments registered for rhizoctonia suppression provide useful but limited control, fungicides applied through in-furrow liquid banding can provide improved levels of rhizoctonia suppression
- Assess root health coming into Spring (see step 4).
Step 3: Ensure quality of planting seed
Seed retained for sowing is a highly valuable asset and the way it was treated at harvest and in on-farm storage during summer, or between seasons, is critical to ensure optimum germination potential and crop establishment in 2020. Retained seed can be tested for vigour, germination, purity/weed seeds and disease pathogens. It is advisable to undertake testing at least two months before sowing so that an alternate seed source can be organised if required. Grading to remove smaller grains which inherently have reduced vigour can also improve the quality of planting seed.
Vigour and germination tests provide an indication of the proportion of seeds that will produce normal seedlings and this helps to determine seeding rates. Particular attention should be given to determining vigour of retained seed for sowing in 2020 due to seasonal conditions in 2018-19. Vigour will be even more important if growers plan to increase sowing depth to capture an earlier sowing opportunity through moisture seeking.
NSW DPI, Tamworth normally provides pathology testing of winter cereal seed for common seed-borne fungal pathogens which will continue in 2020. Germination is also noted but this only tells growers how much of their seed is alive with the main purpose of testing to determine levels of fungal infection present. Testing will be extended for the 2020 pre-season to also provide an indication of vigour and emergence which should be used as a guide only (see contact details).
A comprehensive GRDC fact sheet outlining issues with retaining seed after challenging seasons is available from the GRDC website (see useful resources). The fact sheet outlines how growers can test their own seed. Alternatively, a range of commercially accredited providers of both germination and vigour tests are available.
Seed treatments containing fluquinconazole, flutriafol or triadimenol, can reduce coleoptile length in cereals and cause emergence issues under certain conditions. These active ingredients should be avoided if sowing seed with potentially lower vigour, sowing deeper, sowing into cooler soils, in soils prone to surface crusting or where herbicides such as trifluralin have been applied.
Step 4: Assess infection levels and root health prior to head emergence
Improved agronomy has considerably reduced the impact of rhizoctonia root rot (e.g. early sowing, grass free canola, pulse and pastures, knife point seeding systems and fungicides). These changes in agronomy have resulted in a significant shift in the symptomology of rhizoctonia root rot from ‘bare patches’ due to seedling infection to development of uneven growth in mid-winter due to infection of crown roots when soil temperatures drop to <10°C. Infection can then continue to develop on the crown roots until the crop matures, and can spread to the seminal root system, limiting water uptake in periods of high evapotranspiration and nutrient limitation. Hence, there is the potential for crown root infection by rhizoctonia to go unnoticed in paddocks as wavy and uneven growth is often associated with a range of other factors. This situation can be easily identified with the help of a shovel or spade! Simply dig up some plants around heading, wash soil away from roots and inspect the general root health - paying particular attention to whether the crown roots are restricted with a ‘spear tip’ appearance. Alarmingly, if seasonal conditions have been good prior to heading, crops with significant rhizoctonia infection of crown roots can appear quite normal but have severely compromised root systems. If the season stays wet with milder temperatures, then infected crops can sneak through with minimal yield loss. However, these same crops are likely to suffer dramatically if drier and warmer conditions are predicted during heading and grain filling.
This is a very similar situation to Fusarium crown rot which can also go unnoticed in paddocks until dry and hot conditions during grain filling trigger the expression of conspicuous whiteheads. However, honey-brown discolouration at the base of infected tillers can be used to determine the extent of Fusarium crown rot infection prior to heading. Simply dig up plants (inspect root health at the same time as above), ensure leaf sheathes at the base of tillers are removed and visually inspect for brown discolouration.
Assessing root health and Fusarium crown rot infection levels around heading allows a grower to make an informed decision at this point in time given seasonal predictions (e.g. cutting for hay or silage, reduce further input costs) rather than simply letting the weather dictate the outcome. Although this would be a less than an ideal situation, such tough decisions can still maximise profitability or minimise losses under these scenarios.
Other potential implications of dry conditions – learnings from north NSW in 2019
Dry conditions can also impact on the lifecycle of necrotrophic fungi which cause yellow spot in wheat or net-blotches in barley. We observed this around Croppa Creek in northern NSW in 2019 with spot form of net-blotch (SFNB) in barley crops. Numerous barley crops in a restricted area had decent levels of SFNB lesions on leaves during tillering. This was surprising as the season was relatively dry up to this point with only low rainfall events (<5 mm) since sowing. Rainfall while limited, was accompanied by early morning fogs. These conditions, while not really contributing to yield potential, were enough to meet the 6 hours of high humidity (>80% RH) to initiate SFNB infections on leaves. Interestingly, due to dry conditions the primary infection propagules (pseudothecia) which have a moisture requirement had not matured on 2018 barley stubble. The primary source of infection was mature pseudothecia present on 2017 or even 2016 barley stubble. SFNB was also present in two barley crops sown into wheat stubble which was surprising. However, conidia of the net-blotch fungus Pyrenophora teres formed on collected wheat stubble after 4 days in humid chambers. This supports 2018 disease survey findings where the SFNB fungus was found to be saprophytically infecting wheat crops due to late rainfall in October, coinciding with senescence of lower wheat leaves.
High levels of SFNB were also present in two barley crops in this same region in 2019 where seed was treated with the fungicide Systiva ®. Reduced sensitivity to this SDHI active (fluxapyroxad) was confirmed by the Curtin University fungicide resistance group in net form of net-blotch (NFNB) populations on the Yorke Peninsula of SA in 2019. Pure SFNB isolates collected from these northern NSW barley crops were sent to Curtin University and were shown to have no reduced sensitivity to fluxapyroxad. In our situation we suspect that dry conditions around the seed prevented Systiva from dissolving into the surrounding soil, limiting uptake through the roots and movement through the plant into leaves. Seedlings had established well and their root systems had penetrated into deeper soil moisture which was allowing them to progress, but the top 10 cm of soil was very dry with little visual loss of red pigmentation from the seed treatment on seed coats at the time of inspection.
Conclusions
The perpetual risk as a plant pathologist is the perception that we are always the bearer of bad news or the ‘grim reaper mentality’. Elevated risk of stubble- and soil-borne diseases in 2020 is inevitable given continuing dry conditions which have prolonged survival of pathogen inoculum. However, practical steps can be taken to identify the level of risk and strategies implemented to minimise but not necessarily fully eliminate disease impacts on wheat and barley crops in 2020. Hopefully wet conditions restrict impact of the two most likely cereal disease risks (Fusarium crown rot and rhizoctonia root rot). However, growers and their agronomists need to be prepared to inspect the root health and stem bases of cereal crops around heading to guide some potentially tough but informed decisions. NSW DPI plant pathologists are also available throughout the season to provide support.
Useful resources
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. The author would also like to acknowledge the ongoing support for northern pathology capacity by NSW DPI.
Contact details:
Steven Simpfendorfer
NSW DPI, 4 Marsden Park Rd,
Tamworth, NSW 2340
Ph: 0439 581 672
Email: steven.simpfendorfer@dpi.nsw.gov.au
PREDICTA is a registered trademark of Bayer.
® Registered trademark
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: DAN00213,
Was this page helpful?
YOUR FEEDBACK
