Assessment and treatment of subsurface acidity
Author: Melissa Fraser (PIRSA Rural Solutions SA) | Date: 20 Aug 2020
Take home messages
- Subsurface acidity and stratification are emerging as serious constraints to crop production across SA, NSW, Victoria, and WA.
- Traditional soil sampling strategies can lead to misdiagnosis of subsurface issues; strategic sampling at specific depth intervals is required.
- Lime rates need to be adjusted to account for subsurface pH, changes in soil texture and organic carbon content down the profile.
- Strategic incorporation/tillage can aid the efficacy of lime application for treating subsurface issues; other subsoil constraints (e.g. compaction) should be taken into consideration to maximise treatment impact, along with the risks associated with soil disturbance.
- Options for treating subsurface and stratification issues are being examined in the new GRDC Acid Soils SA Project.
Background
Soil pH is largely a function of soil type, rainfall and farming system, and can be inherently variable both horizontally and vertically in the profile. A soil pHCa between 5.2 and 7.5 provides optimum conditions for most agricultural crops, though plant species differ in their tolerance to acidity (low pH) and alkalinity (high pH).
Acidity can be a severe soil degradation problem that greatly reduces the productive potential of crops and pastures. Acidification is a natural process however, it is often accelerated under productive farming practices, primarily driven by the leaching of nutrients (especially nitrates) from topsoil, and the removal of alkaline farm products. Where no lime is applied, the topsoil becomes acidified and the acidic layer spreads down the soil profile into subsurface layers (Fleming et al. 2020).
The development of acidity can induce nutrient deficiencies and/or toxicities, limit crop responses to fertiliser application and adversely affect root growth and water uptake as toxic amounts of aluminium are released into the soil solution. Additionally, for acid-sensitive crops like pulse legumes, rhizobia survival and nodulation are compromised at pHCa below 5.0, reducing plant vigour and N fixation (Burns et al. 2017a). Acidic conditions also contribute to the suppression of organic matter breakdown and cycling of organic N within the subsurface layer (Paul et al. 2003).
Subsurface acidity is the acidification of the soil below the top 10cm; the delineation between surface and subsurface acidity is important as monitoring and treatment options will vary, becoming increasingly complex at depth. Remedial action is required to curb its development; when it comes to subsurface acidity, prevention is better than cure.
Key Question 1 - How wide-spread is the problem of subsurface acidity and why is it suddenly on our radar?
Much of SA’s 4.4 million hectares of productive farmland has a topsoil pHCa below 5.5 or has the potential to develop acidity (Figure 1). The potential for subsurface acidity to develop across these areas is high, particularly where lighter textured A horizons are thicker than 20cm.
Acidic layers at 5 to 15cm are becoming increasingly common under no-till systems in the high and medium rainfall regions of southern Australia, even where topsoils have been limed (Angus et al. 2019, Burns et al. 2017b, Paul et al. 2003, Scott et al. 2017). The development of these discrete acidic bands is often referred to as ‘stratification’, and commonly occurs at the depth where N fertiliser is applied.
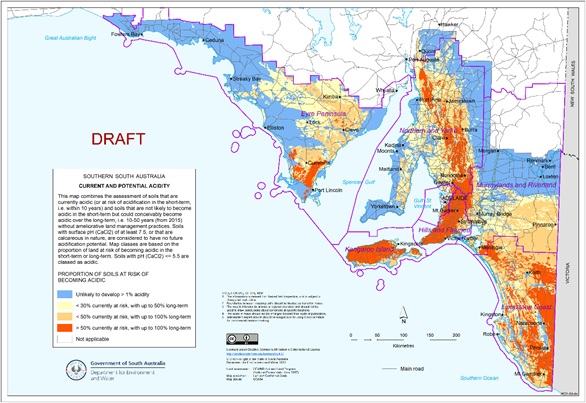
Figure 1. Map of South Australia showing the proportion of soils at risk of becoming acidic (including currently acidic soils). (Source: Department for Environment and Water, Government of South Australia)
In 2019 there were various reports of subsurface acidity and stratification across the State, including in unexpected regions, such as the Murray Mallee and Yorke Peninsula. Across the Limestone Coast, acidity is prevalent both in deep sands and on duplex soils in the north and south, and in the red loams of the eastern border, particularly where the intensity of cropping has increased over the past two decades.
Soil type plays a large part in determining the susceptibility for subsurface acidity to develop. In duplex soils the changing soil clay content, which drives pH buffering capacity, can have an impact on the speed of development of acidic subsoil layers (Paul et al. 2003). The higher soil organic matter content in surface layers may also buffer against pH changes, maintaining a higher pH than the underlying soil. Conversely, the lack of organic matter in light textured sandy subsoils can mean that severe acidity can develop quickly. Given the high spatial variability in soil properties in the region, even at the paddock scale, subsurface acidity is often widespread, but not uniform.
Key Question 2 - How do growers assess whether it’s a problem on their farm or not?
The presence of subsurface acidity is often masked by conventional topsoil sampling methods (0-10 cm), with an often alkaline 0 to 3-5 cm layer diluting acidic bands below, resulting in an overall pH value that doesn’t cause alarm (Figure 2). Where stratification and/or subsurface acidity is present, strategic soil sampling methods are required to accurately detect pH variability and its severity in the profile; targeted sampling to depths at suitable increments is required.

Figure 2. Example soil pit face with pH indicator dye applied. An alkaline surface layer can be seen (purple), overlying acidic soil (bright green) below 3cm.
In the paddock, pH indicator dye can be used to quickly and cheaply determine whether acidity is contributing to poor plant growth.
In the lab, a soil pH test measures the amount of hydrogen ions in a 1:5 solution of soil to water (pHW), or soil to calcium chloride (pHCa). As pH can be affected by soil moisture status and seasonal conditions, it is recommended to measure pHCa. This test is offered by all commercial laboratories and enables test results from different seasons to be more reliably compared.
The pHCa is often 0.5 to 1 unit lower than pHW. To achieve optimum plant growth, the soil pHCa should be maintained above 5.5 in the top 10cm, and above 4.8 in the subsurface (below 10cm), but these threshold values are currently under review and being tested in SA.
Diagnosis
Late winter is a great time to look for acidity issues (along with other subsoil constraints), with patches of poor crop growth often being observed visually and from satellite (NDVI) images, particularly in faba bean and canola crops (chickpea, lentil and barley are also sensitive crop types). Previous yield maps can also often point to areas of ‘good’ and ‘poor’ production in a paddock, assisting with the identification of diagnostic sampling zones.
Summary of process to diagnose acidity issues:
- Access NDVI and/or old yield maps to detect variable plant growth in each paddock. Take these to the paddock to identify diagnostic zones that reflect areas of good and poor production. These areas often align with changes in soil type and topography.
- Within each diagnostic zone, dig 3 to 5 holes to 40cm, creating a flat vertical soil profile face.
- Apply pH indicator liquid dye down the profile and then apply the powder and let the colour develop (Figure 2). Alternatively, you can use a Dig Stick soil probe (spurr probe) to remove an intact soil core and apply the same procedure to assess the change in pH.
- Once the colour reaction is complete, use the diagnostic colour card to determine the pH down the profile. Any acid layers will be visible as bright green or yellow colours. The pH measured with this dye is equivalent to pHW, so the ideal pH is between 6.5 and 8 on the card (Table 1).
- Use a tape measure to identify the positions of any pH changes and take a photo, including the tape measure for future reference.
- N.B. As the indicator solution can deteriorate over time and the observations are visual (subjective), care should be taken with interpreting results.
Table 1. Severity of acidity as determined using a pH indicator kit, which is equivalent to the pH in water (pHW).
Rating | pH |
|---|---|
Neutral | 7 |
Mild | 6.5 |
Moderate | 6 |
Strong | 5.5 |
Severe | 5 and below |
If acidic areas have been identified using pH indicator dye, additional soil sampling and more accurate laboratory pH measurement and other analyses are recommended:
- Within each diagnostic zone, collect 20 to 30 cores, combining the soil from each relevant layer depth in a clearly labelled bucket.
- Depending on the position of the acid layer in the profile, soil depths for sampling might include: 0-5, 5-10, 10-20 and possibly 20-30 cm. If acidity is more common in the 5-15 cm layer, then depths of 0-5, 5-15 and 15-25 cm are more appropriate.
- Thoroughly mix the samples for each layer depth for each zone and bag a sub-sample; send to an accredited laboratory for pHCa analysis, organic carbon % and a soil texture assessment (this information is needed to calculate a lime rate). Aluminium (measured in CaCl2) is also warranted.
Alternatively, precision soil sampling approaches, such as grid-based or on-the-go Veris® pH mapping can provide more detailed data on the variability in surface pH and possible stratification. These maps should still be ground-truthed to assist interpretation, diagnose subsurface issues and generate variable rate lime prescriptions.
Key Question 3 - What are the options to treat subsurface acidity and how important is it to identify other constraints before treatment?
Acidic soils must be limed– lime it or lose it!
Lime treats acidity by neutralising the acid reaction in soils. The carbonate component of lime consumes hydrogen ions in the soil solution and in doing so raises the pH. Lime should be applied at rates to keep the surface pHCa at 5.5 or more in the top 10cm (Burns et al. 2017a, Conyers and Scott 1989, Scott and Conyers 1995).
The rough rules of thumb to change the pH by one unit for each 10cm depth of soil are: 2t/ha of good quality lime for a sandy soil; 3t/ha for a sandy loam; and 4t/ha for a loam/clay loam. Where organic matter is low (common in subsurface layers and/or lower rainfall areas), rates can be substantially reduced and will have the same effect.
However, as lime can come from a variety of sources with different qualities and effectiveness, application rates need to be adjusted to reflect lime quality. If soil magnesium levels are low, consider using dolomitic lime instead to prevent grass tetany in livestock.
Calculators are available to assist with lime rate decisions and assessment of lime quality from different sources on the Acid Soils SA website. N.B. these decision support packages were developed to target surface acidity only (0 to 10cm) and will be reviewed as part of the new SA project to calculate lime rates that account for subsurface acidity.
As lime usually moves very slowly in soils, about 1cm a year at best, incorporating lime through strategic cultivation is recommended when treating subsurface acidity. The more vigorous the soil disturbance after lime application, the faster the soil will be neutralised (Angus et al. 2019).Effective forms of deep cultivation include soil mixing (spading, large offset discs) and soil inversion (mouldboard plough, modified one-way disc plough), with deep ripping (with and without inclusion plates) and delving offering less mixing of applied lime. Cultivation and deep tillage assessments are included in several current trials across the state in the GRDC Acid Soils SA project.
As most soils in the south east region contain a combination of chemical and physical constraints, such as acidity and water repellence and/or compaction, strategic deep tillage and/or soil mixing that extends beyond the top 10cm can be used to alleviate multiple constraints in a single pass. Implementing strategic cultivation/tillage to treat multiple constraints effectively spreads the cost and risk of incorporating lime and maximises the potential gains in production (Davies et al. 2019).
A complete set of methods to diagnose sandy soil constraints in SE SA, along with suitable lime incorporation methods can be found on the MacKillop Farm Management Group website.
Conclusion
Subsurface acidity is becoming increasingly prevalent across SA’s cropping land, leading to patchy plant growth and reduced grain yields, especially in pulses. Its presence often goes unnoticed until it is well developed, due to limited or inaccurate subsurface soil sampling and assessment. A strategic soil sampling approach is proposed to adequately identify stratified and subsurface bands of acidity, particularly in no-till systems. Lime application rates need to be developed that take into consideration the degree and depth of acidity, soil type and organic matter content, and lime quality. Growers should consider methods to incorporate applied lime to increase its efficacy in treating subsurface issues. PIRSA is working on developing new calculators to assist lime rate decisions to treat subsurface acidity and will assess incorporation methods suited to South Australian soils.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
Useful resources
References
Angus J, Bell M, McBeath T and Scanlan C (2019) Nutrient-management challenges and opportunities in conservation agriculture. In (Eds J Pratley and J Kirkegaard) “Australian Agriculture in 2020: From Conservation to Automation” pp 221-236 (Agronomy Australia and Charles Sturt University: Wagga Wagga).
Burns H, Norton M and Tyndall P (2017a). Topsoil pH stratification impacts on pulse production in SE Australia. GRDC Update 2017 Wagga.
Burns H, Norton M and Tyndall P (2017b). Topsoil pH stratification impacts on pulse production in SE Australia. GRDC Update 2017 Bendigo.
Conyers MK and Scott BJ (1989). The influence of surface incorporated lime on subsurface soil acidity. Australian Journal of Experimental Agriculture 29:201-207.
Davies S, Armstrong R, Macdonald L, Condon J and Petersen E (2019). Soil Constraints: A Role for Strategic Deep Tillage. Chapter 8 In (Eds J Pratley and J Kirkegaard) “Australian Agriculture in 2020: From Conservation to Automation” pp 117-135 (Agronomy Australia and Charles Sturt University: Wagga Wagga).
Fleming N, Fraser M, Dohle L and Hughes B (2020). Subsurface acidity: how far has the research advanced? GRDC Update 2020 Adelaide.
Paul KI, Black SA and Conyers MK (2003). Development of acidic subsurface layers of soil under various management systems. Advances in Agronomy 78:187-214.
Scott BJ and Conyers MK (1995). Magnesium nutrition and lime movement down the profile. Making better fertiliser, lime & gypsum recommendations. Proceedings of a workshop. Agricultural Research Institute, Wagga Wagga, Australia, August 15 and 16, 1995.
Scott BJ, Conyers MK, Burns HM, Evans CM and Fettell NA (2017). Stratification of acidity in the shallow soil surface - experiences in the cropping areas of southern and central NSW. In: Proceedings of the 18th Australian Agronomy Conference, Ballarat, Australia.
Contact details
Dr Melissa Fraser
Rural Solutions SA, PIRSA
74 Struan House Rd, Struan via Naracoorte, SA 5271
0427 084 569
Melissa.fraser@sa.gov.au
@Mel_Fraser1
GRDC Project Code: UOA1905-015RTX,
Was this page helpful?
YOUR FEEDBACK
