Insecticide resistance in the green peach aphid (Myzus persicae) and redlegged earth mite (Halotydeus destructor) in Australia – current status and updated management strategies
Author: Babineau M (Cesar Australia), Ward S (Cesar Australia; The University of Melbourne), Arthur A, Maino J, van Rooyen A (Cesar Australia), Hoffmann A (The University of Melbourne) and Umina P (Cesar Australia; The University of Melbourne) | Date: 12 Aug 2021
Take home messages
- In the Australian grains industry, an over-reliance on broad-spectrum insecticides, combined with a limited number of registered chemicals, results in strong selection pressure favouring the evolution of resistance in multiple invertebrate pests.
- The green peach aphid (Myzus persicae; GPA) and the redlegged earth mite (Halotydeus destructor; RLEM) are two important grain pests that have evolved resistance to multiple insecticide groups in Australia. Surveillance demonstrates that resistance is expanding, emphasising the need for a re-evaluation of current management strategies.
- Taking an integrated approach to pest management limits the need for prophylactic insecticide applications by utilising biological and cultural control options, thereby reducing the likelihood of further insecticide resistance evolution.
- Biological management tools include ensuring correct pest identification and the promotion and use of naturally occurring and/or introduced beneficials.
- Cultural management options include creating refuge to reduce pyrethroid resistance in RLEM, sowing into stubble to reduce GPA landing rates, and reducing GPA weedy hosts such as capeweed, wild radish, and dock prior to sowing.
Background
Several important invertebrate pests of grain crops have evolved insecticide resistance in Australia, including in Victoria. These include the green peach aphid (Myzus persicae, GPA) and the redlegged earth mite (Halotydeus destructor, RLEM) (for overview including resistance in other invertebrate pests, see Umina et al. 2019), which have evolved resistance to multiple chemical classes in Australia.
This paper presents the current distribution status of insecticide resistance in the GPA and RLEM in Australia, with a focus on Victoria. Data on the current resistance status of these pests is presented. Current research into novel management strategies utilising modelling, refuge and natural enemies to manage insecticide resistant populations is described. Finally, the most recent guidelines on insecticide resistance management strategies (IRMS) are discussed.
Methods
Green peach aphid
Populations of GPA were collected throughout the grain growing regions during 2019 and 2020. Populations were screened for genetic mechanisms of insecticide resistance to four modes of action: dimethoate (organophosphates Insecticide Resistance Action Committee (IRAC) Group 1B) with esterase E4FE4 ratio, pirimicarb (carbamates IRAC Group 1A) with MACE mutation, alpha-cypermethrin (synthetic pyrethroids IRAC Group 3A) with kdr and super-kdr mutations, and imidacloprid (neonicotinoids IRAC Group 4A) with the gene copy number of the cytochrome P450 gene CYP6CY3. For neonicotinoid resistance testing, a subset of populations was also screened using phenotypic laboratory bioassays. Dose-response curves were generated by plotting percentage mortality against log concentration. Mortality data was analysed using a logistic regression model at each time point. Logistic regression is suited for the analysis of binary response data (i.e., dead/alive).
The occurrence, abundance and field distribution of known aphid parasitoid wasps, many of whom parasitise GPA, were investigated in canola crops (Brassica napus L.) in Victoria (further details in Ward et al. 2021). Briefly, a total of 10 canola fields were surveyed in 2017 and 2018. Monitoring was performed within the field, shelterbelts and refuge for each site. Monitoring was repeated three to six times per season for each field to provide temporal resolution. Four methods were used to detect parasitoid wasps; using yellow pan traps deployed for 24 hours, direct detection following two minutes surveying in the area, using a vacuum for one minute, and identification following the retrieval and rearing of aphid mummies in the laboratory. Aphids were also categorized by species and counted. Wasp identification was validated using molecular barcoding of the cytochrome c oxidase subunit 1 (CO1) gene.
Redlegged earth mite
RLEM populations were collected in grain and pasture growing regions across Australia, between 2011 to 2019 (further details in Arthur et al. 2021). Briefly, populations were collected from fields with reported spray failures and/or fields with a known history of high insecticide and intensive cropping usage. From 2017 to 2019, the majority of populations collected focussed on regions where the recently developed H. destructor models estimated resistance evolution would be greatest. Mite populations were screened against synthetic pyrethroids and organophosphates using phenotypic laboratory assays. Molecular screening was also undertaken to assess pyrethroid resistance by screening mites for the kdr genetic mutation known to confer pyrethroid resistance. The association between pyrethroid resistance and crop management factors was also evaluated using a regression model.
To assess the effect of unsprayed refuge strips on RLEM pyrethroid resistance, field trials and modelling approaches were tested (further details in Maino et al. 2021). The aim was to provide the optimal spatial configuration of susceptible refuges to reduce the likelihood of resistance evolution while minimising total yield impacts. Briefly, a lucerne (Medicago sativa L.) field, located in Tintinara SA, harbouring a low-level (15 % kdr allele) pyrethroid resistant RLEM population was selected. An area of 50 m2 was sprayed with bifenthrin (Talstar®) in order to increase the proportion of resistant individuals in this given area. RLEM samples were collected at three time points (before initial spray, one month after spray, nine months after spray) along transects spanning the sprayed area and 15 m beyond into unsprayed paddocks. Mites were subsequently genotyped for pyrethroid resistance using the kdr allele. Changes in the estimated resistant allele frequency through time was estimated using a general linear model. Finally, a modelling framework for spatially fine-scaled resistance spread and evolution was utilized and updated with the quantified kdr recessiveness rates. Refuge size was then varied in across multiple simulations to observe the effect on canola yield and resistance so that an optimal refuge strategy could be recommended.
Results and discussion
Green peach aphid
Insecticide resistance status
Between 2015 and 2019, a total of 473 GPA populations were genetically screened against known pesticide resistance conferring alleles for carbamates, organophosphates, synthetic pyrethroids and neonicotinoids. This work identified target site resistance in almost all screened populations to carbamates and synthetic pyrethroids rendering these chemicals ineffective as a control option for GPA (Figure 1). Based on these findings, it is recommended growers to do not use either carbamates or synthetic pyrethroids to control GPA in grains crops.
This testing also detected resistance to organophosphates and neonicotinoids in a substantial number of GPA populations (Figure 1). Resistance to organophosphates was found to be moderate in many populations and a result of metabolic resistance. Therefore, organophosphates will provide control in some situations, but less or no control in others. Furthermore, continued use of organophosphates on such populations would likely increase their overall resistance to chemicals from this group. The neonicotinoid resistance inducing CYP6CY3 gene was only found at low copy numbers in the GPA populations screened, which indicates complete chemical field failures are unlikely to occur given the results presented here. Research is continuing to understand the impacts of this resistance on the effectiveness of neonicotinoid-based seed treatments.
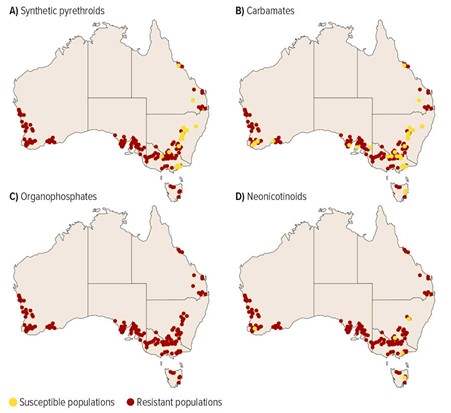
Figure 1. Resistance status of GPA populations tested for resistance to A) synthetic pyrethroids, B) carbamates, C) organophosphates and D) neonicotinoids. The darker coloured dots represent resistant populations while the lighter coloured represent susceptible populations. Source: McGrane et al. (2021).
Parasitoid wasps for control of GPA
GPA was the most abundant aphid species within canola crops at 61 % of total aphids surveyed across canola fields. Interestingly, GPA was not found in shelterbelts and was only found in low proportions (25 %) in refuges. Across all fields, aphid populations remained relatively low during the early stages of crop growth and increased as the season progressed. The most common parasitoid reared from GPA was Diaeretiella rapae (M’Intosh) at 96 % of total wasps reared (Table 1). More generally, D. rapae was the most common parasitoid for all aphid species with an average of 60 % reared from total wasps yet was present only in low abundance at field edges (data not shown). Mummification rate significantly increased from 3 to 4 % in September/October, increasing as crop growth stage progressed and peaking at 20 % by the end of November.
Table 1. Percentage of primary parasitoid species composition of aphid species from fields and surrounding vegetation in 2017 and 2018. Modified from Ward et al. (2021) with permission.
Parasitoid wasp species | Aphid species | ||||
|---|---|---|---|---|---|
Green peach aphid (Myzus persicae) | Oat aphid (Rhopalosiphum padi) | Cabbage aphid (Brevicoryne brassicae) | Turnip aphid (Lipaphis erysimi) | Corn aphid (Rhopalosiphum maidis) | |
n=379 | n=1 | n=62 | n=81 | n=50 | |
Diaeretiella rapae | 96 % | 0 % | 98 % | 60 % | 16 % |
Lysiphlebus testaceipes | 0.2 % | 0 % | 0 % | 6 % | 40 % |
Aphidius matricariae | 0.5 % | 100% | 2 % | 29 % | 24 % |
Aphidius colemani | 0.5 % | 0 % | 0 % | 1 % | 18 % |
Aphidius absinthii | 0 % | 0 % | 0 % | 3 % | 2 % |
Aphidius ervi | 3 % | 0 % | 0 % | 0 % | 0 % |
Canola field edges did not appear to act as reservoirs for parasitoids, as there was little overlap in the community composition of either (data not shown). Location significantly affected the number of parasitoids collected within and surrounding canola fields, with higher numbers collected in the field compared with the grassy refuge (Mean square = 1.27, F = 49.42, p < 0.001).
Redlegged earth mite
Insecticide resistance status
Since the first detection of pyrethroid resistance in RLEM in 2006, resistance surveillance has been undertaken on a yearly basis. Throughout Australia, this has resulted in 1,029 populations being tested over the last 13 years. A total of 195 RLEM populations have now been detected with pyrethroid resistance, 59 populations with organophosphate resistance and 24 populations with resistance to both chemical groups. In Victoria, all populations tested were susceptible to synthetic pyrethroids, and only one population was resistant to organophosphates (Figure 2). Surveillance has covered a wide geographical range throughout eastern Australia, covering a large portion of the distribution of RLEM in this region (Figure 2). Resistance in RLEM is now present across three Australian states (WA, SA, and Vic) and covers more than 3,000 km.
Using field history information, we identified associations for the first time between crop management practices employed by farmers and the presence of pyrethroid resistance. Management strategies that could minimise the risk of further resistance evolution include limiting local spread of resistance through farm hygiene practices, crop rotations and reducing pesticide usage.
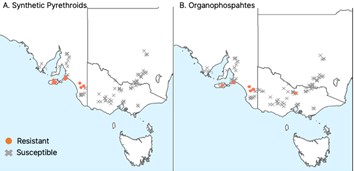
Figure 2. Resistance status of RLEM populations tested for resistance in eastern Australia to A) synthetic pyrethroids, B) organophosphates. Circles indicate resistant populations, while crosses indicate susceptible populations. Image modified from Arthur et al. (2021) with permission.
Novel refuge strategy could delay pyrethroid resistance in RLEM
Experimental field studies demonstrated that a small, localised pyrethroid resistant mite population can revert to susceptibility at farm relevant scales and conditions. Computer simulation results found that certain field configurations (e.g., treatment strip width of 50 m and refuge spacing of 10 m) maintained very low levels of resistance across a 10-year time horizon (Figure 3). Mite population density was also estimated to be lower at this configuration compared with others. At the selected configuration (treatment strip width of 50 m and refuge spacing of 10 m), yield loss is also predicted to be minimal. Interestingly, a larger unsprayed refuge did not always delay resistance in these simulations due to the low migration ability of this pest – i.e., susceptible mites could not move back into the treated areas when both sprayed sections and untreated refuges were large.
Strip spraying to maintain refuges can be readily incorporated into RLEM management programs where sprayer widths in commercial cropping contexts are typically between 20 m to 40 m. A refuge approach to RLEM management that uses strip spraying may enhance long term control options in the absence of new chemical registrations.
This could be a successful strategy to manage RLEM as part of an IPM program. However, this novel approach will require further field validation in a variety of cropping contexts.
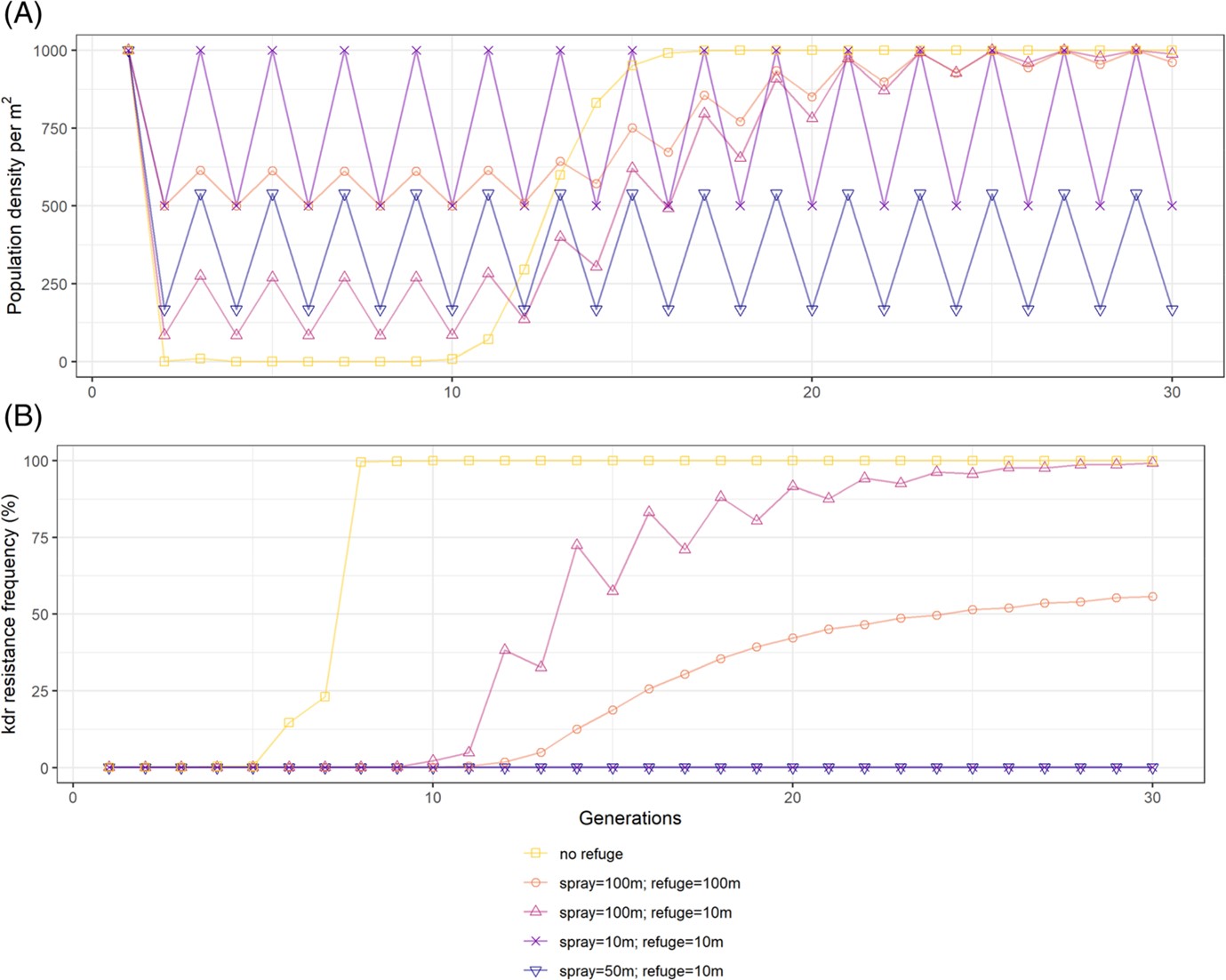
Figure 3. (A) Mean simulated abundance and (B) kdr resistant allele frequency through time under four strip spraying regimes. Source: Maino et al. (2021).
Conclusions
Growers are increasingly facing the challenges posed by insecticide resistance in GPA and RLEM in Victoria. Insecticides will continue to play an important role in GPA and RLEM control, however, the increasing spread and evolution of resistance raises concerns for the long-term viability of chemical control.
Future control of these pests should emphasise an IPM approach that aims to reduce chemical usage to limit selection pressures and decrease the risk of further resistance development. These new strategies should involve the use and conservation of parasitoid wasps against GPA, as well as the use of unsprayed refuges against pyrethroid resistant RLEM.
Resistant management strategies (RMSs) for RLEM and GPA are important resources that help maintain the effectiveness of existing chemistries. RMSs have been developed by the National Insecticide Resistance Management (NIRM) working group for major resistant invertebrate pests in grains. The RLEM and GPA RMSs provide recommendations regarding effective pest management practices. In addition, a recent GRDC investment (BWD1805-006SAX) has helped develop best management practice guides (BPMG) for RLEM and GPA, published in 2020 (see Useful Resources section below). Growers and advisers are encouraged to become familiar with these guides and the RMSs – all freely available to download from the GRDC website.
General resistance management strategies include the following key principles:
- Monitoring crops for pest and beneficial invertebrate presence.
- Accurate invertebrate pest identification to determine the appropriate control strategy.
- Utilising non-chemical control options that suppress invertebrate pest populations.
- Using economic spray thresholds to guide chemical applications.
- If applying multiple insecticides, rotating the chemical mode of action.
- Using selective chemicals, where possible, in place of broad-spectrum options.
- Considering the secondary impacts of chemicals to non-target invertebrate pests and beneficials.
- Complying with all directions for use on product labels including using full recommended rates and good coverage of the target area to ensure the best possible chance of contact and subsequent control of the invertebrate pest.
Acknowledgements
The research undertaken here is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC. We also thank Corteva Agriscience, BASF, ISK, CropLife Australia, and Bayer CropSciences for their support and acknowledge our project collaborators, CSIRO, SARDI, NSWDPI and WA DPIRD. In particular, we acknowledge Andrew Weeks, Xuan Chen, Matthew Binns, Moshe Jasper, Alan Lord, Svetlana Micic, Owain Edwards, Jenny Reidy-Crofts and Sarina Mcfayden. For the parasitoid wasp research, this work was further supported by the Michael Mavrogordato award from the Australian Native Animal Trust, the Albert Shimmins Fund, and the Australian Grains Pest Innovation Program.
Useful resources
GRDC Green Peach Aphid Best Management Practice Guide - Southern
GRDC Green Peach Aphid Resistance Strategy
GRDC Back Pocket Guide Beneficial Insects - Southern and Western
GRDC New knowledge on pests and benefits in grains
GRDC Insect resistance in the southern region
GRDC Fact Sheet Redlegged Earth Mite resistance strategy
GRDC Redlegged Earth Mite - Best management practice guide - Southern
References
Arthur AL, Maino J, Hoffmann AA, Jasper M, Lord A, Micic S, Edwards O, van Rooyen A, Umina PA (2021) Learnings from over a decade of increasing pesticide resistance in the redlegged earth mite, Halotydeus destructor (Tucker). Pest Manag Sci 77, 3013–3024.
Maino JL, Hoffmann AA, Binns M, Cheng X, Rooyen A, Umina PA (2021) Strip spraying delays pyrethroid resistance in the redlegged earth mite, Halotydeus destructor: a novel refuge strategy. Pest Manag Sci p. 6497.
McGrane L, Noakes F, Umina PA, Maino J, Lye J, Arthur A (2021) Pesticide resistance in Australian grain regions – lessons to be learnt. GRDC Update Papers. Grains Research and Development Corporation.
Umina PA, McDonald G, Maino J, Edwards O, Hoffmann AA (2019) Escalating insecticide resistance in Australian grain pests: contributing factors, industry trends and management opportunities. Pest. Manag. Sci 75, 1494–1506.
Ward SE, Umina PA, Macfadyen S, Hoffmann AA (2021) Hymenopteran Parasitoids of Aphid Pests within Australian Grain Production Landscapes. Insects 12, 44.
Contact details
Dr Marielle Babineau
Level 1, 95 Albert St, Brunswick, Victoria 3056
+61 (03) 9349 4723
mbabineau@cesaraustralia.com
@cesaraustralia
youtube.com/cesaraustralia
GRDC Project Code: CES2001-001RTX, CES2010-001RTX, UOM1906-002RTX, CSP1501-002RTX, BWD1805-006SAX,
Was this page helpful?
YOUR FEEDBACK
