A new diagnostic tool for botrytis in chickpeas – in-paddock biosensors - progress towards development of an in-paddock diagnostic device that is cheap, reliable, and accurate
Author: Ido Bar (Griffith University) | Date: 25 Feb 2022
Take home message
A novel, specific and highly sensitive molecular probe-based nano biosensor device and diagnostics protocol were validated for both Botrytis cinerea and B. fabae on field host crop samples.
The diagnosis of the Botrytis spp. was sensitive, specific, quantitative, fast and low cost and allowed the detection of inoculum prior to the visible appearance of disease symptoms.
The tools developed and protocols validated within this work may be applied to many other pathosystems, dependant on the development of target-specific probes and protocol optimisation.
Additional research is required to perform broader in field and in industry validation and to simplify sample preparation and DNA extraction to develop this prototype into a compact and portable device that can inform on the presence, distribution and quantity (inoculum load) of crop pathogens. If done, this would provide significant power to disease management decision making, more targeted (and potentially reduced) chemical usage and potentially improved grower returns.
Botrytis grey mould (BGM), caused by Botrytis cinerea and B. fabae, separately or within a complex, substantially reduces grain legume yield during environmentally conducive seasons. Fast, accurate and cost-effective diagnosis and quantification of the causal pathogen(s) can lead to greater success in application of integrated disease management approaches to reduce yield and profit losses.
Biosensors that use functionalised magnetic gold nanoparticles for molecular target enrichment have been recently developed to detect cancer biomarkers with extreme specificity, sensitivity and accuracy (Islam et al., 2018). In this project we have partnered with experts from the Queensland Micro- and Nanotechnology Centre at Griffith University to adopt this diagnostics approach for the detection of plant pathogens, using Botrytis spp. as a case study.
To achieve this, biotinylated capture probes were developed based on the MRR1 and NEP1 genes B. cinerea and B. fabae, respectively. The probes were assessed for their specificity and sensitivity to detect the pathogens from field collected faba bean leaf samples using a functionalised magnetic gold nanoparticles biosensor assay (Bilkiss et al., 2020).
Sampling of faba bean leaf samples was performed at four field sites in south-eastern South Australia (Millicent, Bool Lagoon, Frances and Mundulla) in October 2020 in a replicated manner. Foliar samples were collected at each site from symptomatic and asymptomatic tissues to provide robust quantifiable levels of target pathogens. Genomic DNA was extracted from five symptomatic and five asymptomatic samples from each site and was used for the biosensor assay.
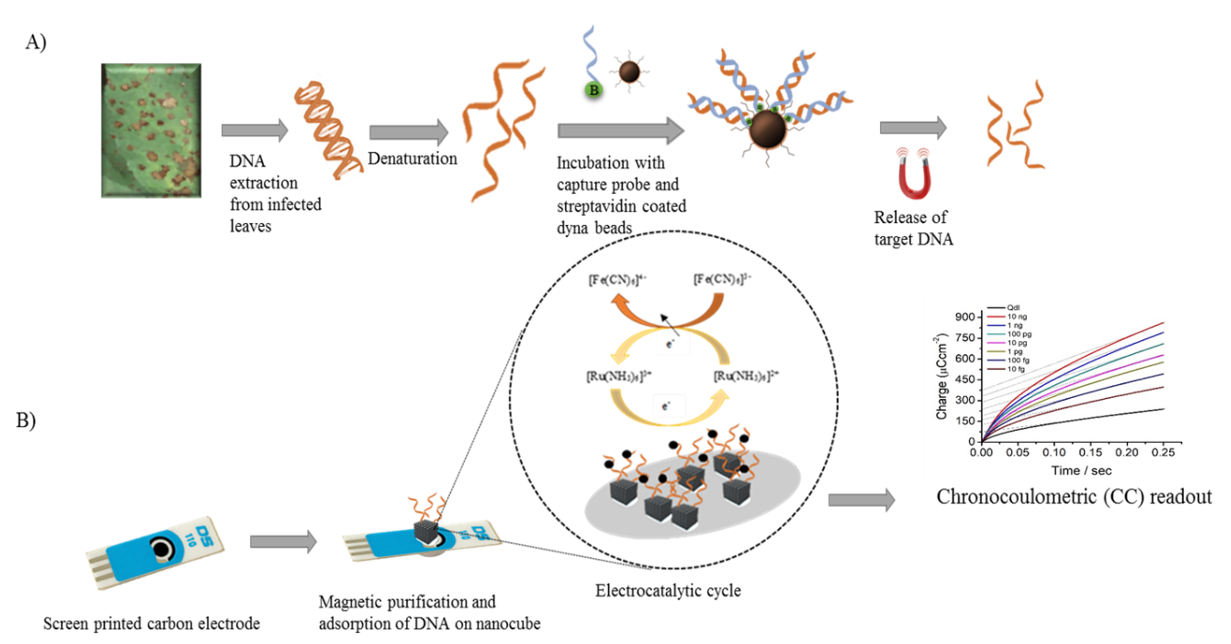
The target DNA was directly adsorbed onto the electrode surface via gold‐affinity interaction, and the amount of adsorbed DNA of each target species DNA was robustly and reproducibly quantified via chronocoulometric (CC) charge density change (Figure 1).
Figure 1. The two-step process for the electrochemical detection of Botrytis spp. from leaf samples. Where A = Magnetic isolation and purification of target Botrytis spp. DNA and B = Electrochemical detection and quantification of the adsorbed target ssDNA (Source: Marzia Bilkiss PhD thesis).
Based on the charge density changes observed, the capture probes were shown to be species-specific to either B. cinerea or B. fabae. The biosensor assay was able to detect single spores of B. cinerea and B. fabae from symptomatic and asymptomatic leaves, thus demonstrating its ability to detect and quantify the causative organisms prior to the visible appearance of the disease on plants and proving to be more sensitive than other published diagnostic methods for both species (Bilkiss et al., 2019). This provides a diagnostic tool for B. cinerea and B. fabae that is highly sensitive, quantifiable, species-specific to each of B. cinerea or B. fabae and fast. The process from sample collection to result is ~45 mins and low cost at <$2 per sample.
The tools developed and protocols validated within this work are open for commercialization partnering through investor engagement. Further investment may be required to perform broader in field and in industry validation and to simplify sample preparation and DNA extraction to develop this prototype into a compact and portable device that provides an accurate and calibrated readout of pathogen loads.
References
Bilkiss M, Shiddiky MJA, Masud MK, Sambasivam PT, Bar I, Brownlie J and Ford R (2020) Electrochemical Biosensor for the Detection of Botrytis spp. in Temperate Legume Crops. International Journal of Bioengineering and Life Sciences 14, 1.
Bilkiss M, Shiddiky MJA and Ford R (2019) Advanced Diagnostic Approaches for Necrotrophic Fungal Pathogens of Temperate Legumes with a Focus on Botrytis spp. Front. Microbiol. 10.
Islam MN, Masud MK, Nguyen NT, Gopalan V, Alamri HR, Alothman ZA, Hossain MSA, Yamauchi Y, Lamd AK and Shiddiky MJA (2018) Gold-loaded nanoporous ferric oxide nanocubes for electrocatalytic detection of microRNA at attomolar level. Biosensors and Bioelectronics 101, 275–281 (2018).
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
We would also like to thank Mohsen Khani and Sarah Blake (SARDI) for their assistance in collecting samples, which was challenging due to COVID-19 travel restrictions, as well as to Muhammad Shiddiky and his group at the Queensland Micro- and Nanotechnology (Griffith University) for their collaboration and assistance developing the biosensors.
Contact details
Dr. Ido Bar
Griffith University
170 Kessels Road, Nathan QLD 4111
Ph: 0435 718 770
Email: i.bar@griffith.edu.au
Prof. Rebecca Ford
Griffith University
170 Kessels Road, Nathan QLD 4111
Ph: 0413 585 439
Email: Rebecca.ford@griffith.edu.au
GRDC Project Code: UOA2007-002RTX,
Was this page helpful?
YOUR FEEDBACK