Will increased canola density change blackleg and/or sclerotinia management decisions?
Author: Steve Marcroft (Marcroft Grains Pathology), Angela Van de Wouw (School of BioSciences, University of Melbourne), Kurt Lindbeck (NSW - Department of Primary Industries, Wagga Wagga Agricultural Institute) | Date: 22 Feb 2022
Take home messages
- Increased canola area will result in increased canola stubble in subsequent years and therefore increased blackleg spore density.
- Spore density can (but not always) result in increased disease severity.
- Increased canola stubble area and the increased area sown to canola will reduce the ability of growers to maintain a 500m buffer between one-year-old stubble and current crops.
- Stubble quantity rather than stubble management has the largest effect on blackleg disease.
- Seasonal conditions will influence whether crown canker or upper canopy infection (UCI) will be more important and potentially warrant control. It will be rare to have severe forms of both versions of blackleg in the same year.
- Crown canker years occur from late sowings, resulting in plants remaining as seedlings during the winter infection period.
- Upper canopy infection years will likely result from early sowing times resulting in plants commencing flowering in late July/early August. Early flowering will result in increased infection and will provide the fungus with more time to cause damage prior to harvest.
- The canola industry is likely to become more reliant on fungicides due to increasing canola production (inability to avoid canola stubble).
- The decision to use a fungicide is not clear cut. You must first understand the disease risk profile of your crop.
- Prior to sowing, use the BlacklegCM decision support tool to identify high risk paddocks and explore management strategies to reduce yield loss.
- Fungicide application for upper canopy infection is a separate decision-making process from crown canker control. Upper canopy infection fungicide application can result in very variable yield returns. You must understand your risk before applying a fungicide.
- Outbreaks of sclerotinia stem rot are sporadic and dependent on the growing season conditions. Saturated canopy conditions for more than 48 hours during flowering favour the development of the disease.
- Current and adjacent paddocks with histories of sclerotinia disease in broadleaf crops over the last four years are a good indicator of potential risk for this season’s crop.
- The frequency of canola or lupin in a paddock is very important in determining the risk of a sclerotinia outbreak, as these crops are very good hosts for the disease and can quickly build up levels of soil-borne sclerotia.
- Foliar fungicides for management of the disease are best applied at 20–30% bloom (15-20 flowers off the main stem) for main stem protection.
Increased canola density will increase blackleg inoculum (spore density). However, inoculum is just one factor that is responsible for disease and disease does not always result in yield loss
Every hectare of canola that you grew in 2021 is now canola stubble. The blackleg fungus survives and reproduces on canola stubble, therefore more stubble results in more blackleg-causing spores. Blackleg is caused by a sexually reproducing pathogen. Individual isolates of the blackleg fungus that have attacked the same plant will survive within the plant and then, on the subsequent canola stubble in the following season, these isolates will mate using the stubble as an energy and structural source. All blackleg fungal isolates are one of two mating types and can only mate with a different mating type. Post-mating, sexual fruiting bodies will appear on the external surface of the canola stubble. The blackleg fungus requires moist conditions for mating and subsequent reproduction. Therefore, this process will occur after the break of the season when the stubble stays moist for a considerable period. Typically, spores will be released from the sexual fruiting bodies once the stubble has stayed moist for 3 weeks. If looking at stubble with a magnifying glass, the fruiting bodies that are still developing look like mountains and fruiting bodies that are releasing spores look like volcanoes. Once the fruiting bodies are developed, they will release spores every time it rains for the rest of the growing season.
As long as stubble stays intact, it will release blackleg fungal spores within the growing season. We have measured spore release in canola stubble for up to four years. However, the blackleg fungus will release fewer spores as the stubble and the fruiting bodies age, that is, one-year-old stubble produces more spores that two-year-old stubble, and so on. The main driver, however, is stubble quantity. Spore release per piece of stubble multiplied by stubble quantity (t/ha) will determine the number of spores that are available to infect your crop. Previous work showed that approximately 99% of spores originate from the previous year’s crop, that is, older stubble produced fewer spores and has less stubble to harbour the disease.
What about stubble conservation
Previous work measured the effect of raking and burning stubble and we even measured spore release after bush fires. We found that deliberate stubble destruction could reduce spore production by 50% but we were unable to measure any reduction in disease severity. There was still enough spores in the raked and burned stubble to drive disease, that is, spores were not the limiting factor. However, it needs to be noted that the raked and burned stubble was still producing vastly more spores per hectare than two-year-old stubble.
Do modern practices of interrow sowing and full stubble conservation change management decisions
Prior to inter-row sowing, canola stubble was knocked down each year via various tillage practices. The stubble lying in contact with the soil, stayed moist during the growing season and released blackleg fungal spores with each rainfall event. Stubble which was two or three years old produced very few spores, so they were highly unlikely to add to the annual disease severity. However, recent work has shown that stubble that remains standing in modern farming practices stays dry, is not developing sexual fruiting bodies at the same rate as the lying down stubble, and therefore, releases fewer spores but the release is later in the growing season.
But how does stubble conservation and interrow sowing impact on disease
During 2021 we undertook experiments comparing disease severity caused by a range of stubble treatments (Figure 1) representing one-year-old stubble or two-year-old stubble that was either lying or standing and with different stubble quantities (low or high). For each stubble treatment, two times of sowing was undertaken to allow for crown canker development (late sown) or upper canopy infection (early sown).

Figure 1. Set up of stubble and plants at Riverside. (a-d) Stubble was set up as either lying (a and c) or standing (b and d) and with either low (a and b) or high (c and d) stubble load. (e-f) Plants were sown in pots at two different times to allow capture of both upper canopy infection (early sown) and crown canker (late sown) infection.
Impact of stubble treatment on crown canker infection
As expected, the earlier sown plants had significantly less crown canker (internal infection), as indicated by average internal infection scores, than the later sown plants, irrespective of stubble treatment (Table 1). For the later sown plants, neither stubble load nor stubble type had any significant impact on disease level with average internal infection ranging between 75.9–92.5%.
Crown canker severity significantly differed across the stubble treatments for the earlier sown plants (Table 1). Significantly more disease was observed in both the standing and lying, high load, one-year-old stubble treatments compared to all others. No significant differences were seen between any standing and lying treatments belonging to the same stubble load. For the high stubble load treatments, one-year-old stubble caused significantly higher levels of disease than the two-year-old stubble.
Taken together, the data suggest that later sowing leads to more significant crown canker compared to early sowing and that stubble load, rather than stubble orientation, has a significant impact on crown canker disease severity. Stubble age, irrespective of stubble orientation, has a significant impact on disease severity but only at high stubble density.
Impact of stubble treatment on upper canopy infection (UCI)
As expected, the earlier sown plants had significantly higher upper canopy infection severity compared to the later sown plants. Very low levels of upper canopy infection were detected in the late sown plants, as expected, with no significant differences between any treatments.
Similar to the crown canker situation, stubble load had a significant effect on disease severity across all stubble treatments for the early sown plants (Table 1). The impact of stubble orientation did cause some significant differences, but only at the low stubble density. Standing one-year-old stubble caused significantly larger stem lesions (34.5cm) compared to the lying one-year-old stubble (8.3cm) at low density, however no differences were detected in branch infection. Similarly, the standing two-year-old stubble caused significantly larger stem lesions (20.4cm) compared to the lying two-year-old stubble (9.6cm). Again, no difference in branch infection was observed.
These data again support the previous findings that earlier sowing times lead to significant levels of upper canopy infection compared to late sowing times. Furthermore, stubble load has a significant impact on disease compared to stubble age or orientation. Stubble orientation did impact the levels of stem lesion infection when stubble was at low density loads.
What does all this mean for stubble management?
- Avoid one-year-old stubble, that is, keep the 500m isolation rule from the previous year’s crop. In this experiment, stubble quantity (high) had significantly higher disease severity for both crown canker and UCI.
- Two-year-old stubble will still produce inoculum and disease but the difference between treatments is minor, therefore the management of two-year-old stubble is probably not warranted.
- The largest effect is time of sowing. Early sown crops get UCI whilst late sown crops are safe from UCI. However, early sown crops are safe from crown canker but late sown crops are vulnerable to crown canker.
Table 1: Effect of sowing time, stubble type and stubble load on crown canker (internal infection) and upper canopy infection (stem lesion length and percentage brown branches). Values within the same disease parameter with the same letter indicate no significant difference (p<0.001). Spore release was also determined from all stubble types and relative proportions determined by comparing total spore numbers to the one-year-old, lying, high load stubble.
Stubble parameters | Disease severity parameters | Spore release | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Stubble age | Stubble orientation | Stubble load | Internal infection (%) | Stem lesion length (cm) | Branch infection (%) | Total spore release | Relative to one-year-old, lying, high stubble (%) | |||
Early sown | Late sown | Early sown | Late sown | Early sown | Late sown | |||||
One-year-old | Lying | High | 49.8c | 85.6ab | 81.0a | 1.7c | 56.7a | 4.5d | 1.9E+08 | 100.0 |
Low | 24.4de | 80.6ab | 8.3c | 0.3c | 27.2b | 3.0d | 1.9E+06 | 1.0 | ||
Standing | High | 54.4c | 84.7ab | 67.6a | 0.4c | 69.1a | 10.9cd | 1.0E+08 | 55.3 | |
Low | 21.3e | 85.9ab | 34.5b | 1.9c | 31.6b | 4.8d | 1.0E+06 | 0.6 | ||
Two-year-old | Lying | High | 36.6d | 80.6ab | 9.6c | 1.3c | 32.1b | 2.2d | 9.1E+07 | 48.4 |
Low | 37.5d | 75.9b | 7.0c | 0.0c | 17.4c | 3.6d | 9.1E+05 | 0.5 | ||
Standing | High | 34.7de | 92.5a | 20.4b | 2.2c | 26.0b | 8.0d | 7.4E+07 | 39.4 | |
Low | 32.5de | 89.4ab | 6.7c | 1.9c | 14.2cd | 4.9d | 7.4E+05 | 0.4 | ||
Your crop is unlikely to get both crown canker and upper canopy infection (UCI) in the same year, therefore you need to know which form of the disease you need to manage this year.
Findings over the past few years have indicated that most years will be defined as a crown canker or UCI year, but rarely both. In most regions, 2021 was a crown canker year. That is, as an agronomist, you will be managing for either crown canker or UCI. The risk is determined by the timing of sowing (germination).
Crown canker
Severe crown canker is most likely to develop when plants are infected during the early seedling stage (cotyledon to fourth leaf). The driving factor for seedling infection is the length of time that the plant is exposed to blackleg infection while in the seedling stage. Therefore, the risk of seedling infection, which leads to crown cankers, is very variable from season to season. Once plants progress past the fourth leaf stage, they are much less vulnerable to crown canker. That is, older plants will still get leaf lesions, but the pathogen is less likely to cause damaging crown cankers as the fungus cannot grow fast enough to get into the crown. Typically, plants sown early in the growing season (April) will develop quickly under warmer conditions and progress rapidly past the vulnerable seedling stage whereas, plants sown later (mid-May) will progress slowly and remain in the vulnerable seedling stage for an extended period.
Upper canopy infection (UCI)
UCI occurs when the plants become reproductive early in the growing season, typically when crops commence flowering in late July/early August. This results in cool moist conditions which are conducive for infection events but also allows enough time for the pathogen to cause tissue necrosis prior to harvest. That is, flower and branch infection (UCI) can occur at any time, but it only results in yield loss if it occurs early in the season. This is because the pathogen must grow from the infection point to within the vascular tissue of the plant where the necrosis occurs, causing yield loss. In 2021, crops that commenced flowering in September in many cases did get UCI but the infection did not progress to the vascular tissue and no yield losses resulted.
Management for increased canola intensity
If you have increased canola production, it is almost certain that you have also increased blackleg severity, however you need to determine if increased blackleg severity will also result in an increased yield loss. If your current farming system has low levels of disease, a small to moderate increase in disease will not be yield-limiting. However, if your crops already have some level of yield loss, increased disease severity will increase the yield loss.
- Know your region – high canola intensity and high rainfall = high risk. One in four-year rotations and 500m isolation between this year’s crop and last year’s stubble reduces risk. Monitor crops for both UCI and crown canker so that you know if you need to retain or change practices.
- Distance to canola stubble – crops sown adjacent to one-year-old stubble will have the highest amount of disease, so maintain a 500m buffer if possible.
- Cultivar resistance – cultivars rated R-MR or above have very low risk of developing crown cankers. MR will develop cankers but only if grown under high disease severity, for example canola/wheat/canola in high rainfall. See GRDC's Blackleg Management Guide.
- Pathogen population – if you’ve grown the same cultivar for a number of years and disease severity is increasing, then you sow a cultivar from the same resistance group, you will be at a higher risk of crown cankers.
- Understand the seasonal risk based on sowing/germination timing – are you managing for crown canker or UCI?
- Fungicides – use the BlacklegCM App to determine potential economic returns for fungicides for crown canker.
- Fungicides for UCI – if your crop has blackleg symptoms and commenced flowering in late July/early August, it is more likely to benefit from a fungicide application. Later flowering crops are unlikely to have yield losses. Cultivar resistance to UCI is effective but we do not yet have a reliable cultivar screening system. If your cultivar has had major yield increases from 30% bloom fungicide applications in previous years, it is likely to be susceptible and benefit from fungicide application.
How does sclerotinia develop?
The complexity of the disease cycle of sclerotinia stem rot results in disease outbreaks being sporadic compared to other diseases. There are several key stages that must be synchronised and completed in order for plant infection to occur. Weather conditions must be suitable for the pathogen at each stage. These stages of development include:
- Softening and germination of soil-borne sclerotia
- Apothecia development and release of ascospores
- Infection of petals by air-borne ascospores
- Senescence of infected petals in the presence of moisture and subsequent stem infection
Weather conditions during flowering play a major role in determining the development of the disease. The presence of moisture during flowering and petal fall will determine if sclerotinia stem rot develops. Dry conditions during this time can quickly prevent development of the disease, hence even if flower petals are infected, dry conditions during petal fall will prevent stem infection development.
What are the factors that drive the development of sclerotinia stem rot (Figure 2)
- Frequency of sclerotinia outbreaks. The past frequency of sclerotinia stem rot outbreaks in the district can be used as a guide to the likelihood of sclerotinia developing this season. Paddocks with a recent history (last 5 years) of sclerotinia outbreaks are a good indicator of potential risk, as well as those paddocks that are adjacent. The frequency of canola and lupin in the paddock can also increase disease risk. Canola and lupin are very good hosts for the disease and can quickly build up levels of soil-borne sclerotia.
- Commencement of flowering. The commencement of flowering can determine the severity of a sclerotinia outbreak. Spore release, petal infection and stem infection have a better chance of occurring when conditions are wet for extended periods, especially for more than 48 hours. Canola crops which flower earlier in winter (late June—July) are more prone to disease development and exposure to multiple infection events.
- Spring rainfall. Epidemics of sclerotinia stem rot occur in districts with reliable late winter and spring rainfall and long flowering periods for canola. These provide long periods of canopy wetness necessary for the disease to develop, at least 48 hours or more. Overnight dews generally don’t trigger epidemics of the disease.
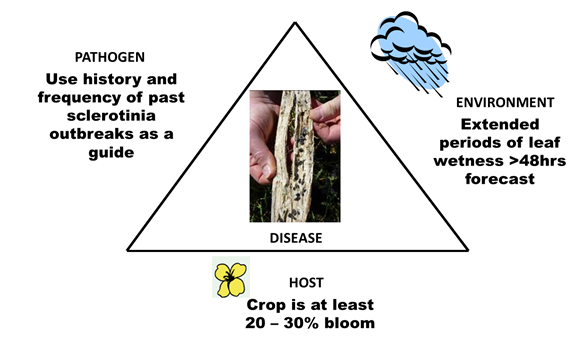
Figure 2. Host, pathogen and environmental factors that drive the development of sclerotinia stem rot.
Key points for low-medium rainfall districts
- Compared to high rainfall districts, serious outbreaks of sclerotinia stem rot will be highly sporadic, once in every 5–7 years, often in years of above average rainfall.
- Background levels of sclerotia are likely to be much less, due to less frequent outbreaks of the disease, reducing disease pressure.
- Shorter flowering periods for canola reduces the opportunity for the disease to develop to damaging levels and crops canopies are less bulky.
- The intensity of canola in the system is often less, reducing high disease risk situations from high inoculum loads.
Pre-sowing sclerotinia management
Crop rotation
- Rotate canola once in every 4—5 years to reduce build-up of sclerotia.
- Incorporate lower-risk crops into the crop rotation, for example, cereals, field pea and faba bean.
- Separate last year’s canola stubble and new seasons’ crops by at least 500m.
- Ascospores spread within 100m to 400m of the apothecia.
Burning
- Burning of stubbles and windrows will kill some sclerotia but will not greatly reduce the risk of disease.
Clean seed
- Always use seed free of sclerotia where possible.
- Grade retained seed for sowing to remove sclerotia if in doubt.
- Grain receival standards allow a maximum of 0.5% sclerotia in the sample.
Variety selection
- There are no Australian canola varieties with known resistance to sclerotinia. Some differences may be observed in the level of stem rot in some seasons. This is likely to be related to the timing of flowering and infection events.
Crop management
- Always follow the recommended sowing time and seeding rate for your region.
- Early maturing varieties sown early can be prone to developing stem infection due to the earlier commencement of flowering when conditions are wet for prolonged periods.
- Once flowering starts, the crop becomes susceptible to infection and prolonged exposure to infested senescent petals means greater chance of stem infection.
- Bulky crop canopies can retain more moisture and are conducive to the development of stem infections.
- Wider row spacing or reduced seeding rates can increase air-flow through the canopy, reducing moisture retention and potential for infection.
Use SclerotiniaCM app (see useful resources) to determine the most appropriate management strategies for your district.
Post sowing sclerotinia management — fungicide application
- Use foliar fungicides to prevent early stem infection via infested petals.
- Always use fungicide products that are currently registered in Australia.
- Timing of foliar fungicide application is more important than choice of fungicide product in reducing potential levels of stem infection.
- Foliar fungicide application is most effective before an infection event.
- Application of foliar fungicide at 20—30% bloom stage is most effective in reducing main stem infection and most yield loss by protecting early petals from infection and penetration of fungicide product into the crop canopy to protect potential infection sites from falling petals.
- Multiple foliar fungicide applications may be needed in high-risk-disease districts with a high yield potential. Applications at both 10—20% and 50% bloom provide critical early and follow-up protection from multiple infection events.
- Use high water rates (at least 100L/ha) to achieve good coverage and penetration into the canopy.
- Foliar fungicides generally have an active life of two to three weeks. The protection provided may wear off during the critical infection period or where crops have an extended flowering period. A single fungicide application too early may be ineffectual.
- Foliar fungicides will have no effect on managing basal infections, as this occurs below the soil surface and beyond the activity of foliar fungicides.
Always
- Determine disease risk as your crop enters the flowering period.
- Assess bloom stage, seasonal conditions and weather forecasts to identify the potential risk to your crop.
- Identify how many consecutive wet days are forecasted as the crop commences flowering and the week ahead, especially consecutive wet days of 48 hours or more.
- Monitor crops for disease development and identify the types of infection. Basal and main stem infections cause the most yield loss.
Useful resources
BlacklegCM App for iPad and android tablets
NSW DPI Winter Crop Variety Sowing Guide (Disease updates, variety resistance, fungicide products)
ScleotiniaCM App for iPad and android tablets
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. The authors also thank Elizabeth Sheedy, Alistair Smith and Buffy Harrison. The authors wish to thank NSW DPI for investment into this research.
Contact details
Steve Marcroft
Marcroft Grains Pathology
Grains Innovation Park
Natimuk Rd, Horsham Victoria 3400
0409 978 941
Steve@grainspathology.com.au
Angela Van de Wouw
University of Melbourne
School of BioSciences
University of Melbourne Victoria 3010
0439 900 919
apvdw2@unimelb.edu.au
Kurt Lindbeck
NSW Department of Primary Industries
Wagga Wagga Agricultural Institute
02 6938 1608
kurt.lindbeck@dpi.nsw.gov.au
GRDC Project Code: UOM1904-004RTX, UOM1306-001RMX, CSP1706-015RMX, MGP1905-001SAX, DAN1703-011BLX, BLG206,
Was this page helpful?
YOUR FEEDBACK
