Where does fertiliser nitrogen finish up?
Where does fertiliser nitrogen finish up?
Author: Peter Grace, Roger Armstrong, Robert Harris, Ash Wallace, Graeme Schwenke and Guangdi Li | Date: 10 Feb 2015
Peter Grace1, Roger Armstrong2, Robert Harris2, Ash Wallace2, Graeme Schwenke3 and Guangdi Li3,
1Queensland University of Technology; 2Victorian Department of Economic Development, Jobs, Transport and Resources; 3NSW Department of Primary Industries.
Keywords: nitrogen, denitrification, N2O, soil organic matter (SOM), volatilisation.
Take home messages:
- Apply nitrogen (N) fertiliser only when the crop can actually take it up, as significant losses can occur via denitrification or leaching when it is sitting in the soil profile doing nothing!
- Increase your soil organic matter levels to provide a slow release of N during the season and reduce your reliance on N fertilisers.
- Reduce N fertiliser inputs by regularly using legumes in your rotations.
- Increase your soil organic matter (SOM) levels as SOM will improve soil structure and drainage and minimise periods of saturation when large losses of N can occur via denitrification.
- Use enhanced efficiency fertilisers to improve nitrogen use efficiency if cost effective.
- Regularly do an N budget to estimate your N fertiliser requirements.
Background
Whilst soil organic matter (SOM) and crop residues are important sources of nitrogen (N) it is widely recognised that this source alone is not sufficient in itself for productive cropping without supplementation from inorganic or organic fertilisers. Nutrient management, especially N, underpins the productivity and profitability of all Australian grain production systems with fertiliser inputs comprising one of the single largest variable input costs for many growers. One tonne of N currently costs in Australia the same as 2.5 - 3 tonnes of harvested grain. Despite its critical importance for on-farm profitability, growers, advisers and researchers alike across Australia still struggle with making ‘the right decision’ about N fertiliser management.
The end game is to increase nitrogen use efficiency (NUE) i.e. the efficiency with which soil mineral N is converted into grain N. The mineral N comes from fertiliser, crop residues, manures, and soil organic matter (SOM), but it is the efficiency of conversion of fertiliser into grain that is generally of greatest concern to growers. Efficiency is reduced by seasonal conditions, crop diseases, losses of N from the soil as gases, N leaching or immobilisation of N into organic forms.
Immobilisation
Immobilisation of N may occur when plant residues of low N content (e.g. wheat or sorghum straw) are decomposing in the soil. Immobilisation represents a temporary unavailability of mineral N in the soil for growing plants to access. Applying fertiliser N in a band separated from crop residues is one means of reducing immobilisation.
Leaching
Leaching is the downward movement of nitrate (NO3-) with water in the soil. Loss of soil N from the plant root zone is more likely in coarse textured (sandy) soils than clay soils. Nitrate is a “mobile” form of N, i.e. can be transported in water through the soil profile, the ammonium (NH4+) form of N is not mobile.
Denitrification
Occurs in saturated soils when nitrate (NO3-) is converted by soil microorganisms into the gases nitric oxide (NO), nitrous oxide (N2O) and di-nitrogen (N2). The loss of mineral N from the soil varies according to conditions and whether there is also a ready source of labile (or readily decomposable) carbon available. Di-nitrogen (N2) is the main form of the gas that is lost, but the proportion of the different gases produced is dependent on soil pH and water content. Denitrification increases as the water content (or water filled pore space – WFPS) increases above field capacity and as oxygen is consumed in the pores. Denitrification occurs more commonly and frequently in heavy textured soils, not coarse textured soils. In acidic soils, more is lost as N2O whereas in alkaline soils most is lost as N2. Once in gas form the N is no longer available to plants in the soil.
The timing of nitrogen fertilisers applied either at sowing, pre-sowing, in-crop or in some combination of these, e.g. some at sowing and some more in-crop as determined by the growing season.
Early application is often more convenient and allows N to move lower into the root zone where it will be accessed later in the season and thus be used more for grain filling than for plant growth, but it is also then at greater risk of denitrification loss should soil saturation/waterlogging occur. Applying N at planting can carry this risk if heavy rains occur before plants begin to use the N from the fertiliser. In-crop applications of N fertiliser require adequate soil moisture on application and/or follow-up rainfall to ensure the applied N taken up by the plant.
Application
Application methods include solid and liquid N products which are applied either directly to the soil surface, placed or injected into the soil (including anhydrous ammonia), or surface applied then covered in a separate operation. In-crop applications are either surface-spread as solids or sprayed as liquids. Surface applications by broadcasting may be at risk of ammonia (NH3) volatilisation, particularly in coarse textured calcareous soils and those with thick stubble layers that prevent the fertiliser from soil contact as it dissolves.
Enhanced efficiency fertilisers (EEFs) are products now being promoted to growers to increase NUE. The EEFs being widely tested in Australia are DMPP-coated urea (commonly known as ENTEC) and Green Urea. DMPP is a nitrification inhibitor which will slows the production of nitrate through the nitrification process i.e. the conversion of the ammonium form of mineral N (produced after the hydrolysis of urea) to the nitrate form. By reducing the amount of nitrate in the soil at any time, mineral N losses via denitrification or leaching may potentially be avoided or significantly reduced. Green Urea contains a urease inhibitor and slows the hydrolysis of urea to the ammonium form of N and potentially reduces N losses via volatilisation.
Results and discussion
To more easily explore the fate of N fertilisers, isotopically labelled (15N) fertilisers have been produced which allow us to track the movement of N applications over the growing season. After harvest, the amount of 15N in the soil, grain and residues is measured and whatever is not recovered is assumed to have been lost to the atmosphere either via denitrification or volatilisation, the latter not being the case in acid soils or if the N fertiliser has been covered or applied below the surface in alkaline soils. Leaching losses may also occur if the N is applied on coarse textured soils.
In the semi-arid temperate Wimmera (annual rainfall 420 mm, alkaline vertosol), 15N fertiliser has been used to explore the interaction between the timing of N application to wheat i.e. 50 kg/ha banded below the seed at sowing or topdressed at GS30 (first node) and three products (urea, Entec, Green Urea). Whilst there was no significant difference in grain yield between the four treatments in both 2012 and 2013 (average 3.8 t/ha), the data collected in the 2013 season confirmed the observations in 2012 that applying urea at GS 30 was the preferred strategy for maximising NUE, as was the use of Green Urea (Figure 1). In 2013, the late application of N fertiliser reduced N losses by 20% (or 10 kg N/ha).
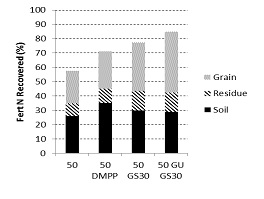
Figure 1. Recovery of 15N labelled fertilisers applied to wheat at Taylors Lake (Wimmera, Vic) in 2013.
A similar observation was made in the High Rainfall Zone (HRZ) of Victoria (annual rainfall 685 mm, acidic chromosol) during the 2012 and 2013 seasons, with extremely high losses of applied N (77- 93 kg N/ha) after deep banding 100 kg 15N/ha of N at sowing compared to a delayed application (Figure 2). On average, 80% of the N applied later in the season was recovered in either the crop or the soil.
The use of the EEF ENTEC increased NUE in the semi-arid environment when N was applied at sowing, but had no impact when used with the later application in the HRZ. The trade-off between the additional cost of EEFs and productivity require further investigation.
At Wagga Wagga (annual rainfall 580 mm, acidic kandosol), the best recovery of applied N in a tillage x N experiment was 77% when only 25 kg 15N/ha was top-dressed (Figure 3). There was no difference in yield between N application rates or tillage treatments (average 3.9 t/ha) and no impact of tillage on total N recovery. The N losses associated with the 25, 50 and 100 kg N/ha treatments were 6, 16 and 36 kg N/ha respectively.
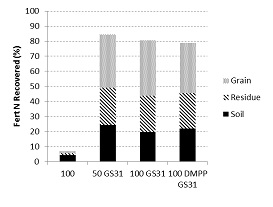
Figure 2. Recovery of 15N labelled fertilisers applied to wheat at Hamilton (HRZ Vic) in 2012.
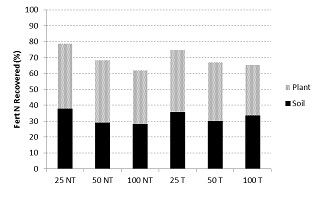
Figure 3. Recovery of 15N labelled fertiliser applied to wheat at Wagga Wagga (NSW) in 2012.
Nitrous oxide
Nitrous oxide emissions are a relatively small loss of N from the soil to the atmosphere, mainly as a result of denitrification during saturation/waterlogging events. Total losses of nitrous oxide (N2O) as a proportion of fertiliser N applied are typically less than 1% in dryland cropping systems but may be higher during prolonged rainfall events and waterlogging, particularly in subsoils which may have restricted drainage.
Nitrous oxide is harmful to the ozone layer and is a greenhouse gas which is 300 times more potent than carbon dioxide in terms of its global warming effect. Small emissions of nitrous oxide are normally indicative of much larger N2 emissions (Figure 4) e.g. for every one kg of N emitted as N2O after seven days of saturation, 52 kg of N as N2 was lost.
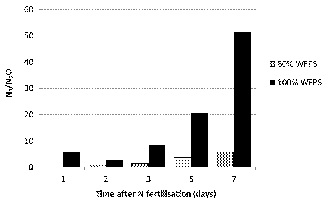
Figure 4. The proportion of N2 gas emitted compared to N2O emitted after from a partially (80% WFPS) and fully saturated (100% WFPS) clay loam soil in Qld (Friedl et al., unpublished).
Ammonia volatilisation
Ammonia volatilisation is the loss of N as ammonia (NH3) from the soil to the atmosphere as a result of chemical reactions at the surface and environmental conditions. Unless it is being applied as anhydrous ammonia fertiliser, ammonia gas is only produced in the soil at a very high pH, which occurs as urea is converted to ammonium N, or when ammonium sulphate comes in contact with calcium carbonate in the soil. Soils with high clay content can buffer changes in pH and also have an affinity for ammonium adsorption, making them less at risk of converting to ammonia gas and being lost.
Ammonia volatilisation data from broadcasted N fertiliser on alkaline soils in northwest NSW is highly relevant to soils in southern Australia. Six different N products have been trialled in four different situations over two years (Figure 5). In three out of four trials on bare fallowed soils without lime, N loss from ammonium sulphate was about half that from urea, urea ammonium nitrate or green urea products. N losses from these products occurred gradually for 2-3 weeks after application of the fertiliser.
In two trials on bare fallowed soils with naturally-occurring lime (at the surface), N loss from ammonium sulphate far exceeded that from urea when applied to bare fallow soils. When N was applied as ammonium sulphate, 37% of the N was lost to the atmosphere. Most of this was lost within the first 2-3 days after application. This is because ammonium sulphate chemically reacts with lime in the soil to form an unstable, high-pH compound that rapidly converts to ammonia gas which can be lost through volatilisation. N volatilisation losses from fertilisers applied as topdressing in-crop (to wheat) tended to be low, averaging just 5% of the N applied.
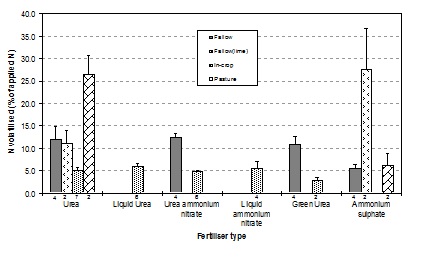
Figure 5. Nitrogen volatilisation losses from surface-applied fertilisers in 15 month-long field trials on clay soils in northwest NSW from 2011-12. The bars indicate the mean N loss across separate paddock trials. The numbers under bars are the number of trial results making up each mean. Fallow (lime) refers to soils with naturally-occurring lime at the surface.
At two grass-based perennial pasture sites, significantly higher N volatilisation losses were measured from urea application compared to ammonium sulphate. Neither of these sites had lime present in the soil. Insufficient rain fell in the week after application to wash the urea through the plant and litter to the soil before it dissolved and began conversion to ammonium. The conversion process, known as hydrolysis, generates a localised high pH that results in more ammonia gas than ammonium ions, resulting in increased N volatilisation losses.
In is important to note that the all of the trials were located on alkaline vertosols, medium to heavy clay soils. N volatilisation was therefore limited by rapid adsorption of ammonium onto the charged clay mineral surfaces, high soil pH buffering capacity, and, when applied in-crop, canopy re-absorption of ammonia, and protection against wind at the surface.
How do these results compare to previous research? Little Australian data is available that has been collected in open field conditions which are critical factors in ammonia volatilisation. Turner et al. (2010) measured 9.5% N loss from urea and 1.0% loss from green urea applied to a wheat crop grown on a clay loam soil in the Wimmera region of Victoria. In another study, Turner et al. (2012) measured an average loss of 18% from urea top-dressed into wheat or barley grown on a grey vertosol in the Mallee. Losses from ammonium sulphate and UAN applied at the same site did not exceed 6%. At another site in the Wimmera, losses from urea totalled 5% and 2% from UAN.
Nitrogen budgets
The amount of mineral N taken up by a three t/ha wheat crop with 12% protein is approximately 130 kg N. In southern Australia, about 40 kg N/ha will slowly be supplied to the crop from stable SOM over one year. The residual effect of the previous season’s crop residues (including roots) on N supply (65-70 kg N/ha for a 3t/ha wheat crop) is negated to some extent by mineralisation during the early (non-crop) months of the year and is susceptible to losses (Figure 6).
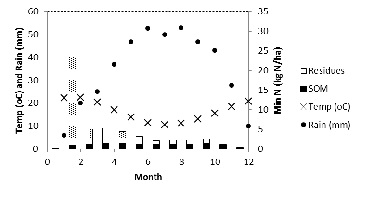
Figure 6. Estimated monthly pattern of N mineralisation from SOM and wheat residues (including roots) in South Australia with 418 mm annual rainfall and topsoil organic carbon (0-10 cm) of 1.5%
Assume that a quarter of the N mineralised from the residues is lost in the first three months (i.e. 15-20 kg N). To close our N budget for the season we need to apply least 40-45 kg N/ha before factoring in any of the loss pathways we have discussed. The 15N studies confirm that top-dressing N is the preferred option with losses of between 20-30% of applied N on relatively well managed soils. The application of 50-60 kg N/ha is therefore considered necessary under these circumstances. These calculations are based on relatively ‘normal’ year. In alkaline soils, the top-dressing must be either covered or coincident with rainfall to minimise volatilisation. The use of Green Urea has been shown to reduce volatilisation without compromising yield but the additional cost of the product needs to be considered.
Conclusion
Improve your nitrogen use efficiency by:
- Only applying N fertiliser when the crop needs it, not before, as large N losses can potentially occur.
- Increasing your soil organic matter levels and regularly using legumes in your rotations as both will provide a native supply of N and reduce N fertiliser requirements and increase profitability.
- Increasing your soil organic matter levels which in turn will improve your soil structure and reduce the amount of time your soils are saturated after rainfall events, and therefore, prone to large N losses.
- Regularly doing an N budget, and/or pre-sowing soil sample, to more accurately estimate your N requirements.
- For every 0.5% of organic carbon (OC) (0-10 cm) approximately 13 kg N/ha of mineral N is supplied from soil organic matter in the top 30 cm over a typical growing season. This N is released slowly over the growing season and not prone to losses.
- For every one tonne of cereal grain, approximately 24 kg N/ha is supplied to the next crop from the crop residues. For every one tonne of legume grain, approximately 55 kg N/ha is supplied to the next crop from the residues. Most of this N is released over the first two months (when a crop is not present) and is prone to loss.
Contact details
Peter Grace
Institute for Future Environments, Queensland University of Technology, Brisbane, Qld 4000.
07 3138 9283
pr.grace@qut.edu.au
