New insights into slug and snail control
New insights into slug and snail control
Abstract
Both snails and slugs can be controlled in no-till, full stubble systems once growers understand the context of where and when controls are applied, and follow a few basic guidelines. Bait needs to be applied when snails and slugs are active and feeding, with the timing varying depending on paddock and seasonal conditions and the species present. Basic rules of thumb for applying bait: snails in autumn once feeding prior to egg laying; slugs at sowing to prevent seed and seedling damage.
Background
The overall aim of research presented in this paper is to improve decisions on bait applications due to the limited field life of current products and the variable feeding of the targets; that is snails and slugs do not always feed on baits. The first aim is to understand factors that lead to the degradation of bait products and compare the field life of various products. The second aim is to understand activity and feeding triggers, and what seasonal factors lead to greater pest numbers. Due to differences in species the research questions have been split with the focus on snails for presentations at the 2016 Adelaide GRDC Grains Research Update and slugs for 2016 Bendigo GRDC Grains Research Update. The subheadings are the current best practice recommendations, with research questions being addressed below that.
Snails
To improve baiting programs apply baits when snails are actively feeding before egg-lay commences
- Under what conditions will snails begin moving and/or feeding?
- Does a snail’s body moisture level change over summer?
- Can we use body moisture to predict feeding and/or time to egg lay?
Slugs
To improve baiting programs apply baits to protect seeds and seedlings from actively feeding slugs
- Under what conditions will adult slugs emerge from soil?
- Can we use soil moisture to predict slug activity?
- Under what conditions and life stage are slugs most damaging to crops?
Methodology
Bait degradation
Italian snails (Theba pisana) were used to test the efficacy of molluscicidal baits once they had been exposed to the environment on soil. Five snails, eight replicates per treatment (n=40) were added to each test arena with eight baits as soon as practicable following the completion of weathering periods (usually within one week). Baits were removed three days after initiation of the experiment due to the formation of mould forming, which was scored as present/absent and the number and condition of pellets remaining recorded. Snail mortality was assessed five days after bait was removed.
Snail and slug activity
Ten paddocks across southern Australia have been intensively monitored using cameras to capture slug and snail activity (A Snug Blog). Environmental data was also collected: soil moisture and temperature (10cm); ground leaf wetness, temperature and relative humidity. Rainfall and barometric data was obtained from BoM. Snails were collected monthly (more frequently during autumn) for assessment of size (n=90), moisture content (n=45) and reproductive stage (n=45) achieved using digital calipers for shell diameter, before and after oven-drying weights (40°C for > four weeks), and albumen gland dissections, respectively. Slugs were collected each month if active from 20 surface refuges per site, which were also used to assess abundance. Slugs were weighed within 24 hrs and dissected to determine reproductive maturity.
Results and discussion
How will the latest research findings affect management strategies and packages?
Research findings regarding the field degradation of baits are presented to inform management about their likely efficacy under various weather conditions. Manufacturers often make claims about rain fastness; however these are based on physical integrity that was found not to influence actual efficacy (Table 1). Conclusions from this work:
- Rainfall erodes physical integrity of bran-based baits.
- Mould on products did not influence bait consumption nor efficacy
- Reduction of a.i. by rainfall (metaldehyde and iron chelate) is important. Individuals are more likely to consume a sub lethal dose.
- Don't use current iron-based baits when >10mm rain is expected
- Temperature, not UV light, degrades metaldehyde baits
- Don't use metaldehyde products over the summer and expect them to last >2 weeks.
- Commonly used bran products need to be re-applied < 2 weeks, more expensive products will last 3-4 weeks
- Work out the cost benefit yourselves!
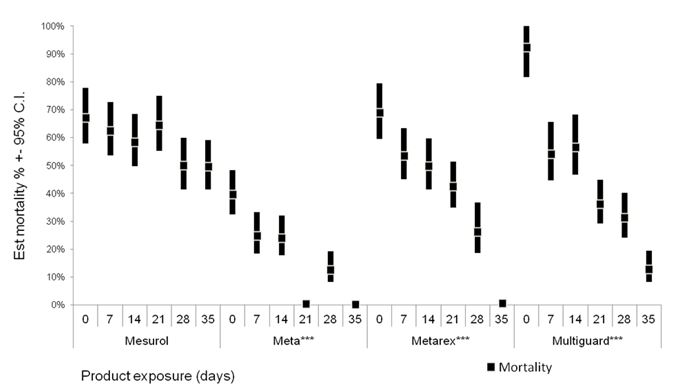
Figure 1: Product degradation in response to exposure to various weather conditions in 2014.
Overall estimates from seven experiments are presented as mean mortality and 95% confidence intervals, calculated from estimating number of dead snails using a generalized linear model (GLM) (Poisson, log link). Significant effect of exposure time is indicated by ***(P < 0.001).
Table 1: Response of various products to rainfall (>35mm) over 2 week period in 2015.
| Product | Active ingredient | Conc. | Different groups no rain | Mortality | Rainfall Sig. Dif. | Rank after Rainfall | |
|---|---|---|---|---|---|---|---|
| 2 weeks | 35 mm | ||||||
| Multiguard | Iron | 6% | a | 97% | 0% | Y | 12 |
| Mesurol | Methiocarb | 2% | a | 94% | 56% | N | 1 |
| Eradicate | Iron | 6% | a | 90% | 0% | Y | 12 |
| Ex 5 | Methiocarb | 2% | ab | 80% | 43% | Y | 2 |
| Ex 1 | Metaldehyde | 3% | bc | 59% | 21% | Y | 6 |
| Ex 3 | Iron | NS | cd | 53% | 0% | Y | 12 |
| Metakill (green) | Metaldehyde | 5% | cd | 51% | 24% | Y | 4 |
| Metarex micrp | Metaldehyde | 5% | cd | 51% | 23% | Y | 5 |
| Ex 2 | Metaldehyde + | 1.8% | cde | 49% | 6% | Y | 9 |
| Ex 4 | Metaldehyde + | 5% | cdef | 44% | 23% | N | 5 |
| Metarex | Metaldehyde | 5% | cdef | 43% | 39% | N | 3 |
| Slugout | Metaldehyde | 1.8% | defgh | 29% | 16% | N | 7 |
| Metakill (blue) | Metaldehyde | 5% | efghi | 24% | 6% | N | 9 |
| Meta | Metaldehyde | 1.5% | ghi | 17% | 7% | N | 8 |
| Sluggoff | Metaldehyde | 3% | hi | 11% | 4% | N | 10 |
| Slugger 2.5mm | Metaldehyde | 1.5% | hi | 7% | 1% | N | 11 |
| Placebo | Nil | 0% | i | 0% | NA | NA | NA |
Combined mortality data (%) from the two experiments (14 reps per group) are presented with significant differences between ‘exposed to rain’ and ‘exposed to dry’ two-week treatments for a single product indicated by different letters. Rainfall resulted in a significant reduction (Χ216 = 658, P <0.001) in efficacy that interacted with product, hence significant differences between individual products is indicated with “Y” (HSD < 0.05), but due to variability we were not able to detect significance when less than a 25 per cent effect size.
Slug research
GRDC fast track project (SAM0001) found the accuracy of bait placement around emerging seedlings gave no significant improvement in protecting the crop from slugs. Canola can be established with disc seeders into stubble in the high rainfall zone (HRZ) as long as some basic rules around timing of bait application are followed. Soil moisture at 50-60cm was associated with increased slug activity at the soil surface as recorded by using surface refuges during this fast track project. Black keeled slug activity is suspected to be triggered by moisture deeper in the soil, hence the current hypothesis is 75mm-100mm of rainfall is required over a three week period in the autumn for populations to become active. Ongoing research is testing this hypothesis and investigating species differences.
Snail activity
Observations, including video footage, have led to more questions than answers. Wetness at 10cm above the ground seems to be best associated with round snail activity, although BoM relative humidity can be used; in summer snail activity is triggered by 90 per cent relative humidity or higher. By late March this response is at 80 per cent relative humidity coinciding with the commencement of mating. Common white snails move more during the night, whereas small pointed snails often move during daylight in the early morning. During wet conditions pointed (conical) snails will move at similar times to round snails. It seems pointed snails (both species) need more moisture to become active, such as longer periods of high humidity or light showers. Observed species differences could be due to temperature, but also behavioural differences. For example, pointed snails have a staggered activity; some are active early in the season before the majority become active mid to late season. One theory is that staggered activity is similar to germination of weeds where buried seeds germinate at a different time to those on the surface. That is, pointed snails in the soil become active at a different time to those hiding up on stubble. This research highlights different species behave differently - bait application needs to match feeding activity.
Snail research
The other observation is that snails often move straight past a bait pellet, making us question claims about pellet attractiveness. Is their response to bait related to their physiological state? That is, does body moisture content of the snail or reproductive stage, as measured by albumen gland size, influence feeding on baits, hence ingestion of a lethal dose. Body moisture content of snails fluctuates during the year in response to available water in the environment. Albumen glands swell during reproductive activity. Enlarged glands indicate active egg-laying. Dissections of two populations of Italian white snails and three populations of common white snails within SA in 2015 indicated that glands began to swell in March 2015, peaking late April to mid-May. Common white snails at Palmer SA displayed a potential relationship between these characters (Figure 2), however a specific body moisture ‘trigger value’ for albumen gland enlargement and subsequent egg-laying is yet to be determined. Incorporating climate data will help predict when body moisture increases, and combined with camera observations the aim is to use site-specific weather data to help pin point optimal baiting periods. In 2015, information from a camera and weather station was used to inform bait application (10kg/ha Metaɸ) in mid-January when snail body moisture was >65% and prior to breeding as indicated by reduced size of albumen glands (Figure 2). This baiting followed rolling in early January, which resulted in >90% mortality. Despite no other bait applied for the season there was limited build-up of common white snails in that 2015 wheat crop.
ɸRegistered label rate is 5-7.5kg/ha.
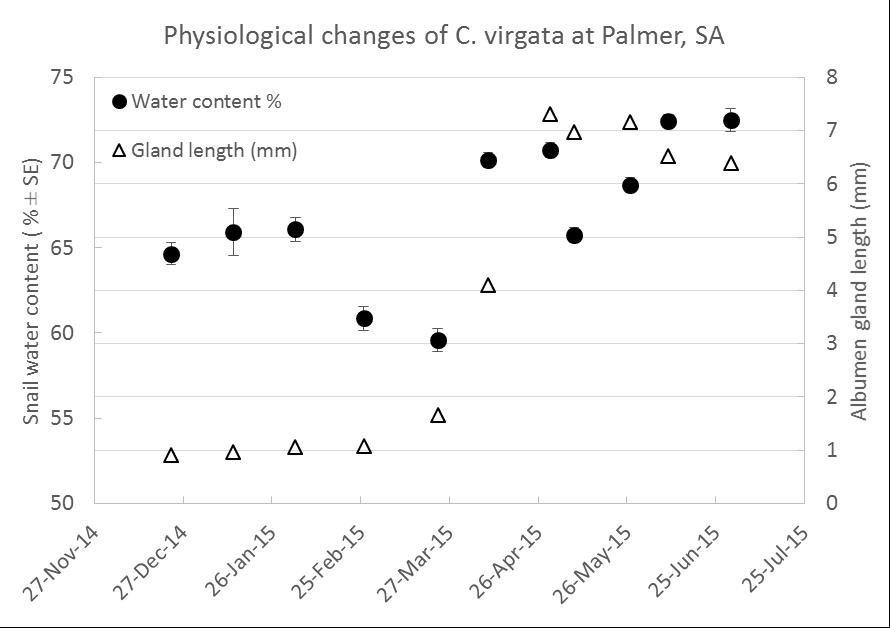
Figure 2: Changes in common white snail moisture and reproductive stage from summer to winter.
Addressing some specific questions that arose from 2015
Where have the slugs gone – does cropping after certain crops (e.g. beans) play a part?
Grey field slug numbers were less in 2015 compared to 2014, however black keeled slug numbers remained constant between seasons (Figure 3). Analysis of camera data indicated that slug activity was reduced during colder conditions, thus damage observed was less in 2015 for most areas. For grey field slugs the question remains: what caused a reduction in their numbers? There were two theories discussed via twitter, email, etc. (i) seasonal conditions meant it was too dry in the 2014 spring for slug numbers to increase, or (ii) the pendulum had swung in favour of natural control, such as an increase in parasites. What is certain is that spring baiting and early autumn baiting prior to sowing rarely prevents damage to emerging crops (Nash et al., 2007); pre baiting does foster complacency.
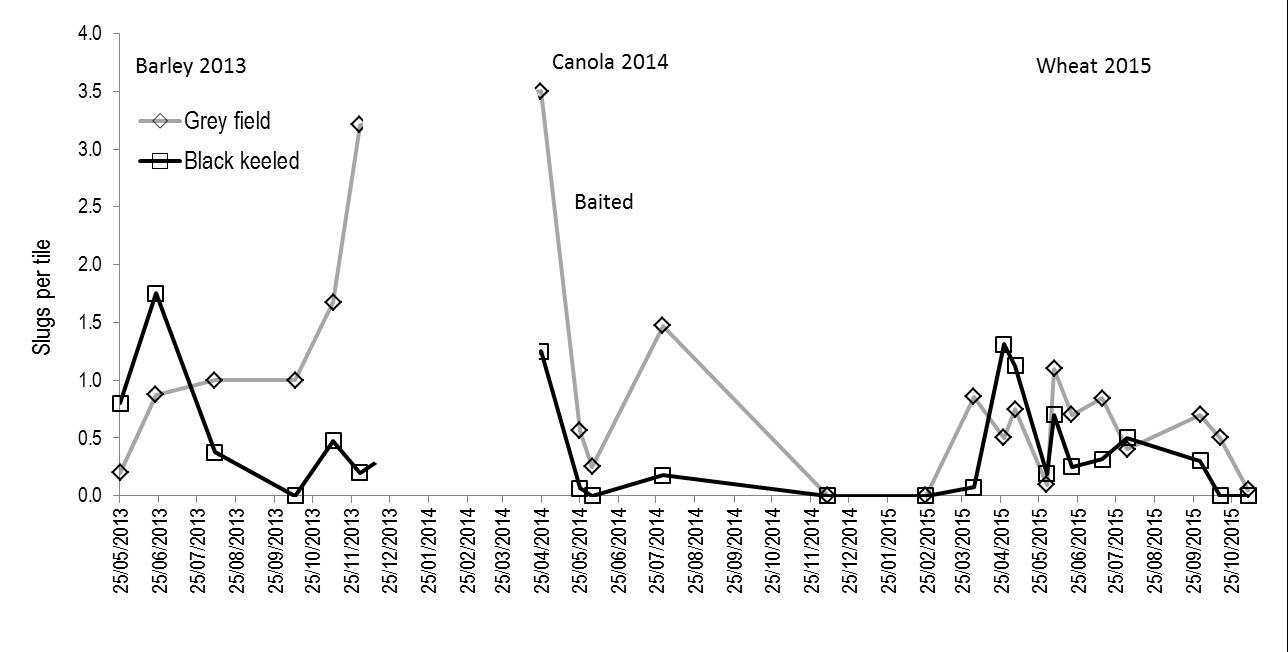
Figure 3: Different slug species relative abundance at one site in western Victoria. Crop type for that season displayed at the top of the figure.
Laboratory experiments were used to determine if nutritional differences between crop types can influence slug abundance and thereby contribute to year-to-year fluctuations in slug populations in the field. Both the number of slugs surviving and the growth rate of individuals differed between six crop types, but most importantly the number of offspring produced was greatest when reared on canola. Peas and canola were the most favourable crop for grey field slugs to increase populations as measured by the intrinsic growth rate (Rm ) (Table 2). With values obtained comparable with previous studies; Rm = 0. 03 (Carrick, 1938, South, 1982). The lack of reproduction on PBA Rana faba beans is not supported by field observations. One likely explanation is, despite beans being a poor food source for slugs (hence beans are easily established where slugs are present), the micro habitat created by bean crops favours slug populations. Interestingly, linseed which had the second lowest intrinsic growth rate has an additional advantage as it is thought to dry out the soil. Preliminary data on volumetric soil moisture from paired paddocks comparing the different 2014 crops, recorded in the winter 2015 were; barley 34.0 per cent, beans 34.1 per cent, linseed 32.7 per cent, canola 34.1 per cent, wheat 41.5 per cent. Significantly lower slug populations (mean ± SE) were observed the following year after linseed paddocks, compared with adjacent barley paddocks: linseed 8 ± 3.4; barley 20 ± 7.3; T test P = 0.014, n=8. Two factors are at play when assessing population responses to crops in the field; microhabitat and nutrition. Investigations are needed to understand why populations increase on wheat, and is this a factor in canola crops being damaged as they follow wheat? Farming groups have a role in extending this laboratory research to the field looking at rotations as a way to limit slug populations.
Table 2: Intrinsic growth rate (Rm) of grey field slugs raised on various crops under laboratory conditions (100% rH, 10-14°C).
| Crop | first eggs laid | eggs | std. dev. eggs | neonates | hatch rate | Rm |
|---|---|---|---|---|---|---|
| Canola | 17/11/14 | 1986 | 192 | 1204 | 61% | 0.026 |
| Peas | 24/12/14 | 1003 | 195 | 530 | 53% | 0.038 |
| Barley | 5/12/14 | 1510 | 182 | 505 | 33% | 0.021 |
| Wheat | 17/11/14 | 2178 | 285 | 492 | 23% | 0.019 |
| Linseed | 5/12/14 | 438 | 56 | 66 | 15% | 0.009 |
| Beans | 5/12/14 | 23 | NA | 0 | 0% | 0 |
What are the risk factors that lead to increasing snail and slug activity?
Improving soils and moisture holding capacity, which includes increasing macropores (porosity), organic matter and available calcium makes a more favourable habitat for snails and slugs. Moisture is the biggest determining factor for breeding, so in seasons that are more favourable for growing crops, populations will build up. Separate tables for slugs and snails have been included for a quick reference to risk factors.
Table 3: Risk factors for slug outbreaks.
| Factor | High risk | Reduce risk | Low risk |
|---|---|---|---|
| Annual rainfall | Irrigated and/or >500mm | 500mm-450mm | <450mm |
| Spring conditions | Above average spring-autumn rainfall | Dry spring hot finish | Drought |
| Establishment conditions | Slow - i.e. cold wet conditions | Quick - i.e. warm dry conditions | |
| Stubble management | No-till, stubble retained | Burnt only | Tilled and burnt |
| Tillage | Press wheels, raised beds, cloddy seed bed | Full disturbance sowing compacted seedbed | |
| Grazing livestock | No sheep in enterprise | Sheep in stubbles | |
| Soil | Soil with improved moisture holding capacity; i.e. increased clay content and organic matter | Poor moisture holding capcity; i.e. sand no OM | |
| Weeds | Summer volunteers | No volunteers | |
| Crop establishment | TT varieties | hybrid varieties canola seed >2mm | |
| Previous paddock history | Slug damage Beans/canola Sclerotinia |
Clean cereal crops | No slugs Poor cereal crop No sclerotinia |
Table 4: Risk factors for snail outbreaks.
| Factor | High risk | Reduced risk | Low risk |
|---|---|---|---|
| Annual rainfall | Above average autumn and summer rainfall | Drought | |
| Stubble management | No till stubble retained | Tillage or burnt only | Tillage and burnt stubbles |
| Grazing livestock | No sheep in enterprise | Sheep on stubbles | |
| Soil | Alkaline calcareous soils | Un clayed non wetting sandy soils | Acid soils with low organic matter |
| Weeds | Summer volunteers/ Brassica weeds Previous paddock history of snails |
No volunteers No history of snails |
|
| Previous paddock history | Snails appear to build up most rapidly in canola, field peas and faba beans but can feed and multiply in all crops and pastures | Clean cereal crops | Poor cereal crop |
Other considerations
Snails
Slugs
Useful resources
Ground Cover TV episodes on slug monitoring and bait timing
References
Carrick, R. 1938. The life history and developement of Agriolimax agrestis L. the grey field slug. Transactions of the Royal Society of Edinburgh, 59, 563-597.
Nash, M. A., Thomson, L. J. & Hoffmann, A. A. 2007. Slug control in Australian canola: monitoring, molluscicidal baits and economic thresholds. Pest Management Science, 63, 851-859.
South, A. 1982. A comparison of the life cycles of Deroceras reticulatum (Muller) and Arion intermedians (Normand) (Pulmonata: Stylommatophora) at different temperatures under laboratory conditions. Journal of Molluscan Studies, 48, 233-244.
Acknowledgement
We would like to thank Jon Midwood and Paul Breust, SFS; Ken Young and Jen Lillecrapp, GRDC; Allan Mayfield, SAGIT; and Felicity Turner, MFMG, for comments and intellectual input.
Funding for this work was provided through the GRDC Project DAS00134 and SAM00001 and their support gratefully acknowledged.
Contact details
SARDI Entomology Unit
GPO Box 397, Adelaide SA 5001
08 8303 9537
michael.nash@sa.gov.au
@merindie1
