Using microwaves to kill weed seeds and snails
Using microwaves to kill weed seeds and snails
Author: John Moore, Senior Research Officer, DAFWA, Albany; Jennifer Westwood, Biosecurity Officer, DAFWA Albany; Svetlana Micic, Entomologist, DAFWA Albany | Date: 24 Feb 2015
Key messages
Microwaves can be used to control seeds, invertebrates and other organisms in soil. This is especially useful for species such as resistant ryegrass, noxious weeds and snails which initially occur in patches, thus allowing more expensive treatments like microwaving the soil to be used.
Microwaves were at least 6 times more effective for killing snails than for killing annual ryegrass seed.
Background
Annual rye grass
Annual ryegrass (Lolium rigidum) is a significant weed of agriculture and has evolved resistance to many herbicides making its control more difficult in southern Australia (Pannell et al. 2004). Research into alternative control methods is essential for the continued control of this weed and others (Gressel 1999). Annual ryegrass is targeted for local eradication where annual ryegrass toxicity or herbicide resistance occurs. The exploration of alternative methods, including direct control of seed banks, is important to the future of weed management. Microwave treatment is more acceptable to organic growers than some other methods. In WA, annual ryegrass is estimated to cause crop losses of $117M/year and at least $33M is spent on annual ryegrass herbicides each year (Moore and Moore 2014).
Depending on the dose, microwave treatment can kill or stimulate the germination of gorse seeds (Moore et al. 2006), Acacias (Cavanagh and Tran 1980) and other species (Bebawi et al. 2007; Brodie 2009).
Brodie et al. (2009) found that both annual and perennial ryegrass germination in the top 10cm of soil was reduced to less than 2.5 percent of that in untreated areas when the soil was wet and the irradiation time was 8 minutes. No seeds within 5cm of the soil surface in wet soil germinated. In dry sand they found that unless the seed was within 2cm of the soil surface there was no significant effect on germination. Germination was also not affected until irradiation was carried out for 12 mins in dry soil, irradiation at this rate reduced germination to 2 percent of that in untreated areas.
High temperatures can kill seeds and microwaves can heat soil or seeds containing water. Lower doses of microwaves may affect protein integrity which may increase or decrease germination.
Snails
Snail management is estimated to cost WA growers $3 million each year in control measures and lost crop production (Murray et al. 2013). A 2013 survey conducted by DAFWA found the small conical (pointed) snail (Prietocella barbara) and the white Italian snail (Theba pisana) were the most common species in WA. These were widespread in coastal areas, especially around Esperance, but also found in significant numbers up to 200km inland.
The white Italian snail was only found on alkaline soils whereas the small conical snail was found on all soil types.
Research into efficacies of baits (see Micic et al. 2013) has found that small conical snails especially immature snails are less likely to take baits than white Italian snails. Consequently, alternative control options for snails are required.
In this study, garden snails (Helix aspersa) were used. The garden snail is a significant problem in horticulture.
Aims
1 -This work investigated the factors that affected the efficacy of microwaves on annual ryegrass seeds to determine a minimum effective dose for control.
It is hypothesised that microwave radiation reduces viability of seed at high doses and increases germination at low doses and these effects will be modified by the seed moisture content at the time of treatment.
2 - To determine the amount of microwave radiation required to give high levels of snail control.
Method
Microwave Radiation of annual ryegrass
Intact seed was soaked for 24 hours in deionised water or left dry, the seed was then placed in glass Petri dishes and microwaved for 0, 10, 30, 60, 120 and 240 s in an 800 watt standard microwave oven, prior to germination. There were 4 replicates of 25 seeds in each treatment. Germination counts were recorded three times a week for 6 weeks after treatment.
Soaked seed (Wet) treatments were analysed using Genstat ANOVA using both the percentage germination and the square root of the percent germination due to unequal variances of the fitted-value plots when using percent germination as the variate. Dry seed treatments were analysed using percent germination as the variate.
Microwave radiation of Garden snails
Active garden snails were collected in Albany and exposed to the microwave treatments on the same day. A single snail was placed in a petri dish and exposed to 1 to 16 seconds of radiation from a domestic 800 watt microwave oven. 8 replicates were taken for the 4, 5 and 6 second exposure times and less for other times when survival was nil or complete. The weight of the snails varied from 2.1g to 8.5g per snail with an average weight of 5.53 ± 1.58g. Shell temperatures were taken with an infra-red thermometer immediately before and after treatment.
Results
Annual ryegrass
Lower doses of microwave radiation were required to kill ryegrass seed that had been soaked for 24 hours before treatment. Dry seed could still be killed but required about 5 times the dose of radiation (see Figure1).
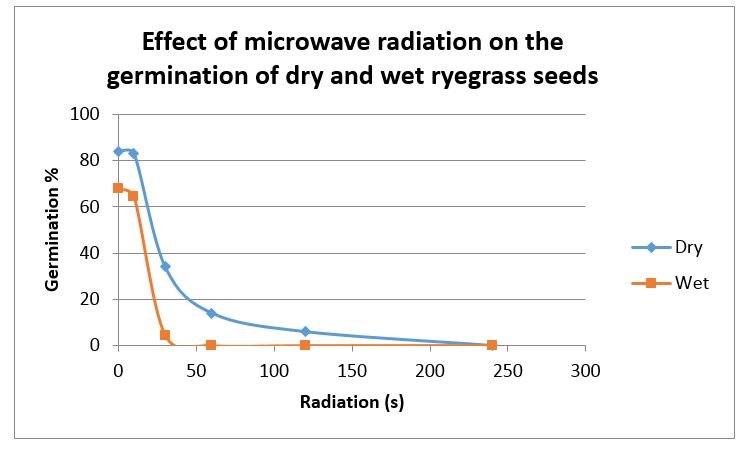
Figure 1: The effect of various doses of microwave radiation on the germination of annual ryegrass seeds that were air dry or were imbibed by soaking for 24 hours before treatment.
Lsd for “Wet” treatment = 2.06. Lsd for “Dry” treatment = 12.9
There was no apparent effect of microwaves altering annual ryegrass dormancy.
Snails
Microwave treatments of 6-7 seconds gave high levels of control of garden snails and this occurred when shell temperatures were around 400C or normal hot summer temperatures. The response to microwave dose was very steep with 4 seconds of exposure causing little damage and 7 seconds resulting in complete death (see Figure 2). Rupturing of internal organs was common above 6 seconds of exposure.
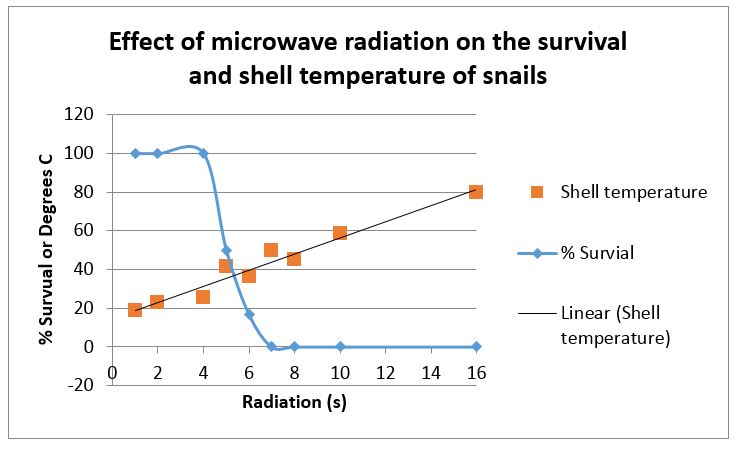
Figure 2: The effect of various doses of microwave radiation on the survival of garden snails and the temperature of their shell immediately after treatment.
Conclusion
The use of microwaves for eradicating small areas of resistant or noxious weeds would be practical with autonomous rover equipment that can move slowly over the area. For ryegrass, with the equipment used here, the exposure time for commercially acceptable control is around 30 seconds. This results in very slow travel speeds unless more powerful microwave generators are used. However for small areas of intractable weeds it still has potential using cheap readily available magnetrons.
For snails only 6-7 seconds of exposure was required for high levels of control. This is also occurring at relatively low temperatures around 400C. This means that there is potential for economic control as the power requirements and the speed of operation is far greater than for weed control. This work needs to be repeated for the small pointed or conical snail and field trials need to be conducted to determine the best configuration of equipment to give the best results as these snails may climb vegetation or bury themselves to avoid hot conditions.
Given these results for snails it should be quite possible to build an autonomous rover that could travel at around 1 km/hr using readily available and cheap magnetrons.
Acknowledgments
Brodie G, Harris G, Pasma L, Travers A, Leyson D, Lancaster C, Woodworth J (2009). Microwave soil heating for controlling ryegrass seed germination. Transactions of the ASABE 52, 295-302.
Micic S, Grimm M, Dore T, Wahlsten L, Learmonth S (2013) Controlling small pointed (conical) snails in southern WA. Crop Updates
Moore (2009) Gorse control in Western Australia (including microwave eradication of seed banks) microwave machines developed by Graham Brodie of University of Melbourne
Moore, C. B. and Moore, J. H. (2014) HerbiGuide - The Pesticide Expert on a Disk. 113. 24.1[24.1]. 1-5-2010. Box 44, Albany, Western Australia, 6331, HerbiGuide, www.herbiguide.com.au.
Murray D, Michael B, Ronning D (2013) The current and potential costs of invertebrate pests in grain crops.
