Herbicide residues in soils – are they an issue? (Northern)
Author: Lukas Van Zwieten, Mick Rose, Pei Zhang, Duy Nguyen, Craig Scanlan, Terry Rose, Gavan McGrath, Tony Vancov, Timothy Cavagnaro, Nikki Seymour, Stephen Kimber, Abby Jenkins, Anders Claassens, Ivan Kennedy | Date: 23 Feb 2016
Take home message
- A national soil survey found that residues of certain herbicides, including glyphosate and its metabolite AMPA, trifluralin and diflufenican, frequently persist at agronomically significant levels in soils prior to the winter cropping season
- Analysis of international literature suggests that soil biological functions are generally resilient to short term impacts of herbicide application at recommended label rates
- However, longer term impacts of herbicide residues, especially after repeat applications, are less well understood. There is evidence that residues at levels found in the soil survey could reduce crop performance, most likely through direct phytotoxicity. The lack of readily available, soil-specific threshold values for herbicide residues causing damage to i) soil biological functions and ii) plant growth is a key knowledge gap to be addressed by future work in this project
- Strategies to avoid herbicide residue accumulation and potential damage to soil functions and crops include: routine rotation of pre-emergent herbicides, reliable record keeping to help identify potential residue issues and organic matter addition to help tie-up bioavailable residues and stimulate microbial activity
Background
The move to conservation tillage and herbicide-tolerant crop cultivars means that many farmers are relying on herbicides for weed control more than ever before. Despite the provision of plant-back guidelines on herbicide product labels, site-specific factors such as low rainfall, constrained soil microbial activity and non-ideal pH may cause herbicides to persist in the soil beyond usual expectations. Because of the high cost of herbicide residue analysis, information about herbicide residue levels in Australian grain cropping soils is scarce.
In addition, little is known about how herbicides affect soil biological processes and what this means for crop production. This is especially the case for repeated applications over multiple cropping seasons. In Australia, herbicides undergo a rigorous assessment by the Australian Pesticides and Veterinary Medicines Association (APMVA) before they can be registered for use in agriculture. However, relatively little attention is given to the on-farm soil biology – partly because we are only now beginning to grasp its complexity and importance to sustainable agriculture. Although a few tests are mandatory, such as earthworm toxicity tests and effects on soil respiration, functional services provided by soil organisms such as organic matter turnover, nitrogen cycling, phosphorus solubilisation and disease suppression are usually overlooked.
GRDC recently co-funded a 5-year project (DAN00180) to better understand the potential impacts of increased herbicide use on key soil biological processes. This national project, coordinated by the NSW Department of Primary Industries with partners in WA, SA, Vic and Qld, is focused on the effect of at least 6 different herbicide classes on the biology and function of 5 key soil types across all three grain growing regions.
Here we report on the results of a field survey of herbicide residues in 40 cropping soils prior to sowing and pre-emergent herbicide application in 2015. We discuss the relevance of these residues to soil biological processes and crop health, with a focus on those herbicides most frequently detected. Recommendations are given to minimise potential impacts of herbicide residues on productivity and soil sustainability. We also detail plans for future research and the development of management tools for growers to monitor and predict herbicide persistence in soils.
Methods
- Farm survey data from the GRDC Focus Paddocks project (DAW00213) was collated and analysed to understand herbicide use practices in the WA grain growing regions. The Focus Paddocks project monitored the farming practices, soil properties and crop yields of 180 paddocks spanning the WA wheat-belt for 5 years. Spray records were converted to quantity (as kg of active ingredient) per hectare and ranked in terms of frequency of application for different crops.
- A soil survey was undertaken to provide a representative snapshot of herbicide residue levels in cropping soils at the beginning of the 2015 growing season (April-May), prior to the application of pre-emergent herbicides. Soil samples were taken from 40 paddocks around Australia, including 12 in WA, 15 in SA, 10 in NSW and 3 in Qld. Composite samples (12 subsamples) were taken from a randomly chosen 50 m by 50 m grid in each paddock, at two depths (0-10, 10-30 cm). Samples were analysed for 15 commonly used herbicides using advanced analytical techniques developed and validated specifically for this project.
- Herbicide impacts to soil biology were reviewed by searching the literature using the search terms herbicide AND soil AND (microb* OR function*). Over 300 peer-reviewed publications were analysed for potential impacts of herbicides on soil organic matter turnover, nutrient cycling and disease interactions.
- The potential for direct phytotoxicity to crops was assessed by comparing herbicide residue to literature thresholds for herbicide sensitivity. Because such data are lacking for glyphosate residues in soil, we also conducted a bioassay to determine the effect of soil-borne glyphosate residues on wheat, lupin and canola growth in a sandy (tenosol) soil from Wongan Hills, WA. This soil has low phosphorus buffer index (for Colwell P) of 15 L kg-1, indicating a low potential for P sorption. Glyphosate (as Roundup CT ®) was thoroughly mixed through topsoil (5 cm) at rates equivalent to 0.33, 1, 3, 9 and 27 times the label rate, and aged for 1 month in the glasshouse prior to sowing. In addition, we tested whether the application of 20 kg ha-1 of P (as potassium phosphate) would alter the toxicity thresholds by re-mobilising soil-bound glyphosate. Root and shoot biomass was measured after a 6-week growth period.
Exposure – which herbicides are being applied?
Despite farmers and advisers keeping spray records for individual paddocks, aggregated (i.e. industry-wide) data for herbicide use practices are not readily accessible. Information collected in the Focus Paddock Project provides a useful snapshot of which herbicides are being used frequently in different crops (Figure 1). Information is also being collected through the National Paddock Survey Project and will be analysed over the coming year.
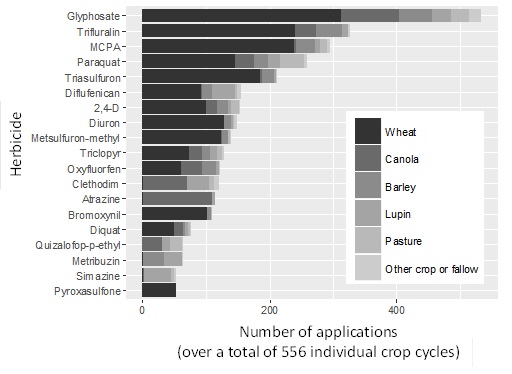
Figure 1. Herbicide use patterns for WA cropping systems. A total of 556 paddock records were obtained in the Focus Paddock survey over the 5 year period.
Glyphosate is the most frequently applied herbicide product in the WA Focus Paddocks, and in all likelihood, other Australian grain cropping regions as well. Glyphosate was used in all crop types/sequences in the WA Focus Paddocks. Given that glyphosate was applied over 500 times to 556 individual crop cycles, this equates on average to almost 1 application per crop. Other commonly used herbicides included those from Group D (trifluralin), Group I (MCPA; 2,4-D; triclopyr), Group L (paraquat), Group B (triasulfuron; metsulfuron-methyl), Group F (diflufenican) and Group C (diuron). Atrazine and glyphosate were the most common herbicides used for weed control in canola. The use of pyroxasulfone (Sakura®) has increased in response to herbicide-resistance.
Exposure – which herbicides are remaining in soil?
The soil survey of 40 different paddocks from around Australia (12 in WA, 15 in SA and 13 in NSW-Qld) detected residues of 11 chemicals out of the 15 analysed (Figure 2). Glyphosate and its primary metabolite, aminomethylphosphonic acid (AMPA) were the most commonly detected residues, with AMPA residues present in every topsoil sample taken. Trifluralin residues were also detected in over 75% of the paddocks surveyed, both in topsoil and in the 10-30 cm soil layer, indicating some vertical movement despite the strong tendency of trifluralin to remain close to the site of application. This is possibly the result of cultivation. However, leaching or movement of particle-bound trifluralin may also occur on lighter textured soils with low organic matter content. Diflufenican and diuron residues were frequently detected in samples from WA paddocks, but less so in NSW-Qld and SA.
Interestingly, despite known application of triasulfuron and metsulfuron-methyl in many of the surveyed paddocks, neither of these residual herbicides was detected in any of the samples tested. This is probably a reflection of their low rates of application, close to the limit of analytical detection. It should be noted that sulfonylurea (SU) herbicides may still have some residual activity at levels below the limit of (currently available) analytical detection. By contrast, the lack of positive detections of frequently applied MCPA reflects its relatively short persistence.
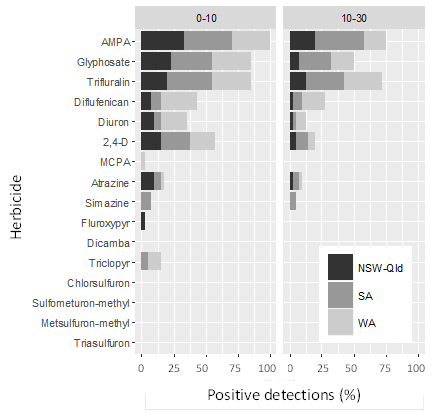
Figure 2. Number of positive detections of herbicides and the glyphosate metabolite AMPA in soil samples from 40 grain cropping paddocks around Australia.
By multiplying herbicide concentrations (mg/kg) by soil bulk density (kg/dm) and area, we estimated the total load of herbicide in the 0-30 cm soil profile for each paddock (Table 1). The average and maximum estimated loads of glyphosate, trifluralin, diflufenican and diuron were all significantly higher in paddocks in WA compared with those in SA, NSW and Qld. This likely reflects the lighter soil types, lower organic matter, dry summers and cool winters, which contributes to lower microbial activity and constrained herbicide breakdown. The higher load of atrazine in SA paddocks is probably a consequence of the higher persistence of s-triazine herbicides in alkaline soils; whilst the higher values for 2,4-D in the NSW-Qld soil profiles was due to a high value in a single paddock which had recently been sprayed.
Notably, in a number of paddocks (especially in WA but also in other states), we found glyphosate in quantities greater than expected from a single spray. This demonstrates a degree of accumulation of glyphosate and its metabolite AMPA over time. Although the half-life of glyphosate is relatively rapid (10-40 days), a significant portion of the glyphosate (and AMPA) is bound to soil and is much less accessible for continued degradation. This, combined with the high frequency of glyphosate use, can lead to a build-up of glyphosate and AMPA in soil. Accumulation of trifluralin was also apparent in a number of paddocks in WA. It should be reiterated that these levels represent the total loads, accessible by aggressive chemical extraction, rather than the bio-available fraction. Aging of residues in soil results in stronger binding over time, and a reduction in bioavailability, so any biological effect can be difficult to predict. This is discussed in more detail in the following sections.
Table 1. Residue loads (average and maximum) of herbicide active ingredients (a.i.) in the 0-30 cm soil profile of paddocks by region.
|
Herbicide |
Estimated average load across all sites (kg a.i./ha)* |
Estimated maximum load detected (kg a.i./ha)* |
||||
|
NSW-Qld |
SA |
WA |
NSW-Qld |
SA |
WA |
|
|
AMPA |
0.91 |
0.95 |
0.92 |
1.92 |
1.97 |
2.21 |
|
Glyphosate |
0.56 |
0.48 |
0.79 |
2.05 |
1.05 |
1.75 |
|
Trifluralin |
0.08 |
0.11 |
0.53 |
0.14 |
0.26 |
1.34 |
|
Diflufenican |
0.01 |
0.03 |
0.04 |
0.02 |
0.05 |
0.09 |
|
Diuron |
0.14 |
0.05 |
0.17 |
0.16 |
0.05 |
0.29 |
|
2,4-D |
0.20 |
0.02 |
0.01 |
1.00 |
0.05 |
0.02 |
|
MCPA |
0 |
0 |
0 |
0 |
0 |
0 |
|
Atrazine |
0.02 |
0.03 |
0.02 |
0.03 |
0.05 |
0.02 |
|
Simazine |
0 |
0.04 |
0 |
0.00 |
0.05 |
0 |
|
Fluroxypyr |
0.03 |
0 |
0 |
0.03 |
0 |
0 |
|
Dicamba |
0 |
0 |
0 |
0 |
0 |
0 |
|
Triclopyr |
0 |
0.04 |
0.01 |
0 |
0.07 |
0.01 |
|
Chlorsulfuron |
0 |
0 |
0 |
0 |
0 |
0 |
|
Sulfometuron-methyl |
0 |
0 |
0 |
0 |
0 |
0 |
|
Metsulfuron-methyl |
0 |
0 |
0 |
0 |
0 |
|
|
Triasulfuron |
0 |
0 |
0 |
0 |
0 |
0 |
*Calculated by multiplying mass concentration (mg/kg) detected by area and average bulk density (derived from soilquality.org) for each soil layer
Toxicity – how do soil functions respond?
A literature review of over 300 published studies identified common themes with respect to herbicide impacts on soil function (Rose et al., 2016). The majority of papers reported negligible impacts of herbicides on beneficial soil functions when applied at recommended rates. Even in the cases where negative effects were observed, they were usually minor and only lasted for periods of less than one month.
However, some exceptions were apparent, especially regarding the effects of repeated herbicide application. For example, there is evidence that the accumulation of some SU herbicides after repeat application can reduce plant-available N, by slowing down the processes controlling N-cycling. Persistence of SUs in soil has also been linked with increased incidence of Rhizoctonia diseases in cereals and legumes. These effects are more likely to occur in alkaline soils, where SU herbicides are significantly more persistent. There are also cases in which other herbicides (e.g. glyphosate) can increase the incidence of disease, but these interactions appear to be site-specific and often occur under stressful growing conditions.
Based on this information and the herbicide residues detected in the soil survey, it is unlikely that SU residues are having ongoing negative impacts to soil functions in the paddocks surveyed. However, the high residue loads of glyphosate, its metabolite AMPA and trifluralin may be altering some soil functions or plant-pathogen interactions. The localised nature of interactions with glyphosate, and the lack of specific data on trifluralin, means that firm conclusions cannot yet be made with respect to the residues detected.
Toxicity – how do plants respond?
Because the potential for each herbicide to damage crops varies according to soil, agroclimate and crop, comprehensive phytotoxicity thresholds (given as soil residue concentrations) for assessing plant-back risk are not readily available. Here we focus only on the potential for glyphosate (+AMPA) or trifluralin residues to cause seedling damage, given their high frequency of application and detection in the residue survey.
It is generally accepted that glyphosate is deactivated when it reaches the soil and poses little risk to crops. However, recent research has shown that under certain circumstances glyphosate can be remobilised and become plant bioavailable, including:
- In the event of P fertilisation, which can compete with glyphosate for binding sites on soil and remobilise bound glyphosate residues (Bott et al., 2011)
- In the event of glyphosate applied to a high density of weeds soon before sowing, such that dying weeds translocate glyphosate into the soil and act as a more soluble pool of glyphosate to the germinating crop (Tesfamariam et al., 2009)
We used a sandy, low organic matter soil from Wongan Hills, WA, to construct dose-response curves for wheat and lupin encountering glyphosate residues applied one month prior to sowing. To demonstrate circumstance (1), half the test pots received a one-off application of 20 kg/ha P fertiliser (as soluble potassium phosphate) at sowing.
As can be seen in Figure 3, in soil not receiving P fertiliser, wheat biomass was not affected by levels of glyphosate in soil resulting from a 27 kg/ha application rate, whilst lupin biomass was only significantly reduced at rates above 12 kg/ha (when upper 95% confidence level falls below 100% biomass). When P fertiliser was added at 20 kg P/ha, both wheat and lupin showed signs of phytotoxicity at lower glyphosate concentration – for lupin this occurred at levels of glyphosate > 3.5 kg/ha (Figure 3, visual growth shown in Figure 4); and for wheat > 12.5 kg/ha (Figure 3). Previous research has shown that increasing levels of P fertiliser application will continue to lower the phytotoxicity threshold to glyphosate/AMPA residues in soil. We are currently analysing the soil samples from this experiment to determine the residue level of both glyphosate and AMPA in soil. This will give us a more accurate understanding of whether the residues found in the field survey are likely to cause crop growth impacts following P fertilisation.
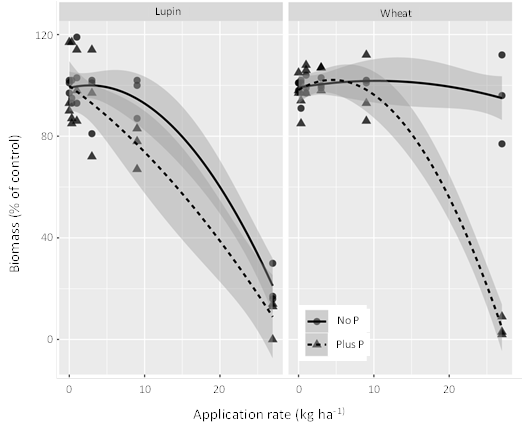
Figure 3. Growth response of lupin and wheat to glyphosate applied to soil one month prior to sowing. P fertiliser (20 kg/ha) was added at sowing to half the pots.

Figure 4. Growth response of lupin to glyphosate applied to soil one month prior to sowing. Note the impact of P fertiliser on lupin growth at glyphosate application rates 9 kg/ha.
With respect to trifluralin, phytotoxicity thresholds for oats vary from 0.1 – 0.2 mg/kg and wheat vary from 0.2 – 0.4 mg/kg depending on the soil type (Hager and Refsell, 2008). Table 2 shows the number of paddocks in which the topsoil trifluralin residue concentration exceeds the lower threshold for oats and wheat, respectively. Again, it must be stressed that the residues detected in our field survey constitute “aged” residues which are likely to be less bioavailable and hence less phytotoxic to crops. Nevertheless, considering that some of these paddocks will receive a pre-emergent application of trifluralin in 2016, the risk of some phytotoxicity is tangible.
Table 2. Number of paddocks exceeding trifluralin lower phytotoxicity thresholds for oats (0.1 mg/kg) and wheat (0.2 mg/kg) in topsoil (0-10 cm)
|
Region |
Trifluralin > 0.1 mg/kg |
Trifluralin > 0.2 mg/kg |
Number of paddocks surveyed |
|
WA |
10 |
5 |
12 |
|
SA |
2 |
0 |
15 |
|
NSW-Qld |
0 |
0 |
13 |
Where to from here?
Ideally, growers and advisers would have tools available for rapid diagnosis of herbicide residues in soil, together with information of the biological relevance of these residues. Our current work is testing rapid in-field dipstick technology (similar to pregnancy test-kits) that can give a semi-quantitative indication of herbicide residue levels in soil within 30 minutes. We are also formulating improved models that can account for the effects of weather and soil type on herbicide persistence, to give growers and advisers the ability to estimate soil residue concentrations in a given paddock at a certain time after herbicide application. Output from current and future glasshouse dose-response experiments on herbicide impacts to soil functions and plant growth will be linked to model output in a handheld, ‘App’ format for quick reference.
Conclusions
- Glyphosate, trifluralin and diflufenican are routinely applied in grain cropping systems and their residues, plus the glyphosate metabolite AMPA, are frequently detected at agronomically significant levels at the commencement of the winter cropping season
- The risk to soil biological processes is generally minor when herbicides are used at label rates and given sufficient time to dissipate before re-application
- However, given the frequency of glyphosate application, and the persistence of trifluralin and diflufenican, further research is needed to define critical thresholds for these chemicals to avoid potential negative impacts to soil function and crop production.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. We would also like to acknowledge the generosity of all farmers participating in this survey for support, access and information regarding on-farm herbicide use. Special thanks to Cindy Cassidy, Tony Pratt and Kellie Jones from Farmlink, NSW; Greg Butler from SANTFA, SA; Clare Johnston and Elly Wainwright from Liebe Group, WA; Sarah Hyde and Georgia Oliver from Facey Group, WA; and Ashley Webb, Annabelle McPherson and Clarence Mercer, NSW DPI, for invaluable assistance with soil sampling and liaison.
Contact details
Lukas Van Zwieten
NSW Department of Primary Industries
1243 Bruxner Hwy, Wollongbar NSW 2477
Ph: 02 6626 1126
Fx: 02 6628 6051
Email: Lukas.van.zwieten@dpi.nsw.gov.au
® Registered Trademark
GRDC Project Code: DAN00180,
Was this page helpful?
YOUR FEEDBACK
