Recent insect pest management research findings and the application of results in the field
Author: Melina Miles, Adam Quade, Richard Lloyd and Paul Grundy, Queensland Department of Agriculture and Fisheries | Date: 23 Feb 2016
Take home message
- Green mirids do cause spotting on faba bean seed. Both adults and late instar nymphs are damaging and warrant control in faba bean crops. No threshold is available yet.
- Preliminary thresholds for Helicoverpa in canola are proposed, based on research that showed the consumption rate per larva to be 2.4g of grain.
- Knockdown products currently used for Rutherglen bug (RGB) are either more effective, or as effective, as a range of ‘new’ products screened. Residual activity still being investigated.
- Cultivation shows promise as an option for reducing high densities of scarab larvae.
- The interaction between scarab density and efficacy of seed dressings and in-furrow treatments (IFT’s) needs further investigation.
Recent research outcomes
Confirmation of green mirid damage potential in faba beans
A replicated trial that caged mirid adults and nymphs on developing and maturing pods was conducted in 2015. The purpose of the trial was to establish conclusively whether mirid feeding caused spotting on the seed, and whether mirids warranted further research as a pest of faba beans.
Pods at two development stages were included in the trial, 1) fully filled, but still green and 2) immature, seed approximately 30% filled. Either 2 adult mirids, or 2 late instar nymphs were confined on a pod for 10 days, and there were control pods on which there were no mirids. At the end of the 10 days the mirids were removed from the ‘cages’ and the cages replaced to protect the pods from being damaged by any other insect or weather. Cages remained on the pods until the pods were mature and dried down (harvestable). During this time cages were checked, and treated where necessary for small nymphs that hatched from eggs laid by the mirids in the trial.

Figure 1. Effect of green mirids caged on faba bean pods of different ages
As shown in the figure above, mirids did consistently cause spotting on the seed coat. Adult and late instar mirids caused similar damage. When filling pods were exposed to mirids, a significant proportion of seed did not develop. This trial result clearly demonstrates the damage potential of mirids in faba beans, both in terms of quality (spotting) and yields (small seed).
This trial was not designed to address the question of threshold. We cannot extrapolate from these data to estimate how many mirids will cause significant seed spotting or screenings. These are areas of research that will be addressed next winter.
Preliminary threshold for helicoverpa in canola
To date, thresholds available for managing helicoverpa in canola have been based on ‘best guesses’. In 2015 we conducted a replicated trial to determine the consumption rate of helicoverpa larvae in canola. A consumption rate is a vital component of an economic threshold, and is an estimate of the yield and crop loss likely to occur. It is calculated from the lifetime consumption of a larva. In other words, it is a reflection of the amount of yield loss that would occur if the larva was not controlled.
The trial involved confining individual larvae on canola racemes and allowing them to feed until they pupated. These data were compared with data from racemes that had no helicoverpa feeding. In summary, results of the trial are:
i) The consumption rate of a helicoverpa larva is estimated to be 2.4 grams of grain per larva.
ii) On average a larva damaged 10.5 pods and consumed 124 seeds.
iii) Larvae showed no preference for pod size/maturity
If we use the following equations, we can calculate the potential yield loss and economic thresholds for a range of crop and cost of control values – presented in the tables below.
Potential yield loss (t/ha) per larva = D x P x V
Where
- D = estimated yield loss per larva (t/ha)
- P = pest density per sampling unit (e.g. per m2)
- V = crop value ($/t)
Economic threshold (larvae/m2) = C / (V x D)
Where
- C = cost of control ($/ha)
- V = crop value ($/t)
- D = estimated yield loss per larva (t/ha)
Table 1. Potential yield loss ($/ha) from helcoverpa larvae in canola
|
Crop value ($/t) |
Number of larvae per m2 |
|||
|
1 |
2 |
3 |
4 |
|
|
400 |
9.60 |
19.20 |
28.80 |
38.40 |
|
450 |
10.80 |
21.60 |
32.40 |
43.20 |
|
500 |
12.00 |
24.00 |
36.00 |
48.00 |
Table 2. Economic threshold (larvae per m2) of helicoverpa in canola
|
Cost of control ($/ha) (application + insecticide) |
Crop value ($/t) |
||
|
400 |
450 |
500 |
|
|
20 |
2.1 |
1.9 |
1.7 |
|
25 |
2.6 |
2.3 |
2.1 |
|
30 |
3.1 |
2.8 |
2.5 |
|
35 |
3.6 |
3.2 |
2.9 |
|
40 |
4.2 |
3.7 |
3.3 |
These thresholds are preliminary, and as it usual in the development of thresholds, the next stage is to repeat the trial as well as to evaluate the effectiveness of the proposed thresholds in adequately preventing economic yield loss in commercial crops. It is expected that we will have greater confidence and firm thresholds within 1-2 seasons.
The other aspect of helicoverpa management that has not yet been addressed, and is critical to the use of economic thresholds, is a reliable sampling method. This will be addressed this coming winter.
Rutherglen bug in canola stubble
Despite their apparent hardiness in the field, Rutherglen bug (RGB) have proven to be very difficult to maintain in laboratory bioassays. We have conducted a number of bioassays to evaluate the efficacy of a range of insecticides that we considered useful either as knockdown products, or with potential as barrier treatments to limit movement into summer crops from canola stubbles. To date we have screened 11 products for knockdown efficacy, and none of the ‘new’ products were superior to those currently used.
Problems with keeping RGB alive for more than 4 days in the lab, has limited our ability to evaluate the residual efficacy of the products. We will pursue this again in 2016.
What is now clear from agronomist observations and our monitoring of canola fields is that the RGB are present in the canola crops as they are filling pods and maturing. The RGB lay eggs in the soil (up to 400 eggs per female), resulting in an explosion of nymphs in 2-4 weeks after the adult RGB are seen. Although not typical, nymphs will move up the plants and onto the pods. More commonly they remain on the soil and base of plants. Where canola is windrowed, the nymphs may move to shelter under the windrows and feed on the shed seed. The risk of RGB damage to seed through direct feeding is not well understood and warrants research. Overseas research suggests that the risk of impact on grain (oil content/quality) decreases as the crop matures and dries down.
Scarabs damaging summer and winter crops
Scarab damage to establishing crops has been reported from the Darling Downs to Northern NSW. The highest number of reports of scarab infestation, and crop loss caused by scarabs, has been from the eastern Downs, but this may be a result of a higher level of awareness in these areas. Sorghum is the crop most frequently reported with crop loss, but crop loss has also been confirmed in sunflower, maize, mungbean, and wheat.
Species and lifecycle
At least 4 species of larvae have been collected from fields. In the majority of cases, there is a predominance of one species in affected fields. Whilst a definite identification is still not available (identification can only be made from beetles, and the beetles are only active for a short period between Oct-Mar), we believe the most common species is Othnonius batseii, the black soil scarab.
Other species of beetle are currently with a scarab taxonomist for identification.
Scarabs generally have a 1-2 year lifecycle, which can be longer if the larvae do not have suitable growing conditions (too dry, inadequate food source). This means larvae can be present in fields 12 months of the year. The larvae feed on roots, impacting on plant growth and ability to tolerate moisture stress. The impact of scarab feeding is visible as slowed crop growth, plant death (often in patches), delayed maturity and lodging.
Damaging densities
Whilst we do not yet have a defined threshold, observations of crop loss and infestations indicate that densities above 15 larvae per sqm (to 15 cm depth) are associated with significant crop loss. This tentative threshold is influenced by a number of conditions including crop, scarab species, soil moisture, cultivation, sowing equipment, use of seed dressings and in-furrow treatments. At present, we think that at densities below 15 per sqm, seed dressings and in-furrow treatments may provide some protection. At higher densities, particularly those above 25 larvae per sqm, observations suggest that even at-sowing treatments do not prevent significant crop loss.
Management options
Cultivation
Two replicated trials have been conducted, comparing the impact of a single offset disc and chisel plough on scarab larvae densities. The results from these trials showed that cultivation, either with disc or chisel plough, reduced larvae numbers significantly (Figure 2). In these trials the cultivation resulted in a full disturbance of the soil surface and correspondingly high reductions in soil moisture. Subsequent sorghum planting at the Cecil Plains site did not establish well in the cultivated plots. We have monitored the impact of several commercial fields where the growers have cultivated infested patches in the field. The results from these fields have shown similarly large decreases in the larval populations.
The impact of cultivation on soil moisture is a major impediment to the potential uptake of cultivation to manage high density infestations. However, it may be possible to be more targeted with cultivation and achieve the same outcome. Examination of the distribution of larvae across the plant row and inter-row shows a concentration of larvae on the plant row in the majority of fields (Figure 3). This pattern of distribution opens up the possibility of more targeted tillage, with reduced disturbance. This area of work clearly requires further research and development.
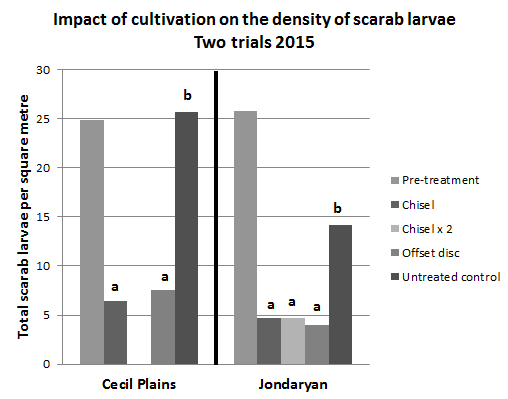
Figure 2. Cultivation with an offset disc or chisel plough reduced scarab larvae densities significantly. Two passes with the chisel plough (Jondaryan only) did not further reduce larval numbers. Means with the same letter above the bar are not significantly different from each other.
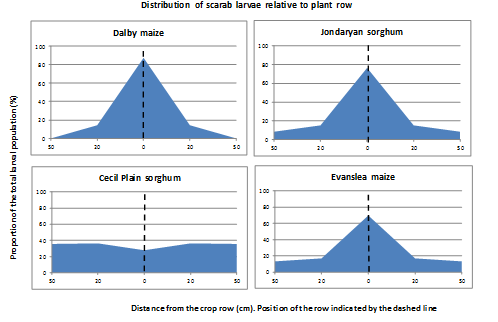
Figure 3. Distribution of scarab larvae in crops on 1m row spacing, showing the concentration along the plant row in three of the four examples
Insecticide treatment
Once a crop is planted, there are no insecticide options for controlling scarab larvae. Consequently, any attempt to mitigate the impact of larvae either by killing them or repelling them from the root zone must be implemented at sowing.
In 2015 we evaluated a range of in-furrow treatments (IFT) and seed treatment options to see what benefit they may be in establishing sorghum in a heavily infested field (average of 26 larvae per square metre). As discussed above, it is likely that there are larval densities at which even effective seed treatments and IFT will not provide adequate protection. However, the trial results (Figure 4) show significant increases in seedling biomass at 53 days after sowing (DAS) with a number of treatments. At the time of writing, no in-furrow or seed treatments have registered label claims for the control of scarabs. The plots treated with two seed dressings (C and D) showed significant increases in biomass compared with the untreated controls. In combination with IFT, there was a further increase in biomass with some treatments.
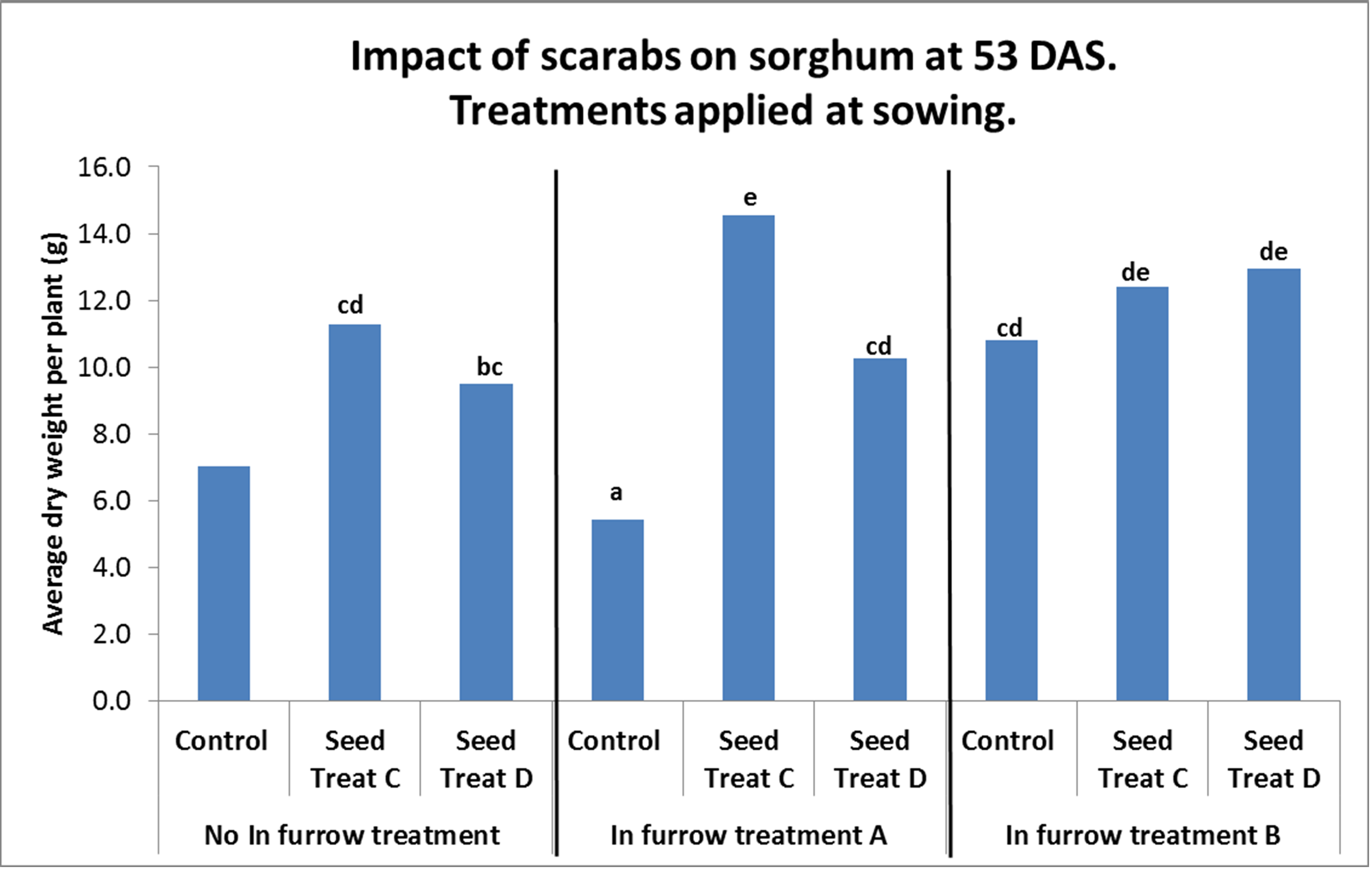
Figure 4. Scarab larva impact on seedling sorghum (to 53 DAS) was significantly reduced with the application of some in-furrow and seed treatment combinations.
We also saw a significant yield increase in wheat, in the same field, in a trial evaluating the efficacy of seed treatments in winter crops.
The use of IFT and seed dressings for a long-lived pest like scarab larvae is not simple. It is probable that the insecticides will deter larvae from the zone in which they are active, but as roots grow out of the treated zone they may then be damaged by larvae. These interactions need investigation.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
We are extremely grateful for the generous support of many growers and agronomists who have allowed us to sample their field or undertake trial work, and made us aware of insect pest issues affecting them. In particular, we acknowledge the assistance and opportunity for field work and discussion provided by James Ryder, John and Paul Griffiths, Glenn Milne, Belinda Chase, Mike Balzar, Steven Hegarty (Vanderfield), Graeme Sutton (Bayer) and Trevor Philp (Pacific Seeds). Much of our field work would not be possible without the assistance of the field staff at the Hermitage Research Station and the Regional Research Agronomist network. The DAF Biometry team, in particular Kerry Bell, has provided valuable assistance with the analysis of trial data.
Contact details
Melina MilesQueensland Department of Agriculture and Fisheries
203 Tor St, Toowoomba, Qld.
Ph: 0407113306
Fx: 07 46881199
Email: melina.miles@daf.qld.gov.au
GRDC Project Code: DAQ00196,
Was this page helpful?
YOUR FEEDBACK
