Wheat rust in 2015 – where are we heading?
Author: Steven Simpfendorfer, NSW DPI Tamworth | Date: 23 Feb 2016
Take home message
- Stripe rust has not gone away!
- Know the difference between a ‘hot individual plant’ and a ‘hot-spot’ before creating panic.
- If you had stripe rust in your EGA Gregory in 2015 it is likely a seed purity issue. Consider freshening up your seed source.
- EGA Gregory remains MR to stripe rust and does NOT require fungicide application
- Consider ‘up-front’ or early season fungicide management of stripe rust in Suntop in 2016, especially under higher nitrogen status
- Be aware of the development and spread of new wheat leaf rust pathotypes in your region
- The north is on track with rust management, do not slip on minimum disease standards, any perceived short-term gains are likely to result in long-term pain for ALL.
Stripe rust in 2015
Stripe rust first appeared in wheat crops in north NSW/southern QLD (North Star and Goondiwindi) in moderately susceptible (MS) varieties (Sunzell and Gauntlet) at the start of August 2015. Cooler autumn/winter temperatures and rainfall during this period were very conducive to the development of stripe rust in 2015. Stripe rust infection occurs as long as there is leaf wetness of between for 5-6 hours (minimum 3 h) with temperatures below 20⁰C (optimum 6⁰C to 12⁰C). During much of the growing season these conditions usually occur overnight. There were numerous reports of stripe rust ‘hot-spots’ in the MR-MS variety Suntop across regions in 2015. Samples of stripe rust were submitted to the Australian Cereal Rust Control Program (ACRCP) at the University of Sydney’s Plant Breeding Institute throughout the 2015 season, with pathotypes 134 E16 A+ (WA pathotype), 134 E16 A+ 17+ (WA Yr 17+ pathotype) and 134 E16 A+ 17+ 27+ (WA Yr 17+27+ pathotype) confirmed in Queensland and northern NSW.
Three non-fungicide treated GRDC funded NVT trials were conducted in northern NSW (North Star, Spring Ridge and Tamworth) in 2015. Early and very high levels of stripe rust developed in the North Star and Tamworth sites with lower and later development of stripe rust occurring at the Spring Ridge site. All trials allowed good evaluation of the relative resistance of wheat varieties and advanced breeding lines to stripe rust in the absence of fungicide protection.
All sites were exposed to natural infection from stripe rust. That is, they were not artificially inoculated with stripe rust spores. The development of significant levels of stripe rust at all three geographically spread sites highlights that stripe rust inoculum was not a limiting factor in the 2015 season. All rusts (stripe, leaf and stem) are biotrophs which means they require a living host to survive between seasons. This is primarily volunteer wheat in the case of cereal rusts but wheat stripe rust has been shown to also survive on barley grass in some seasons. Barley grass also gets infected by a barley grass specific stripe rust pathogen that cannot infect wheat but can cause infection on some barley varieties. Barley stripe rust is not currently present in Australia which is fortunate, as overseas screening indicates that around 80% of current barley varieties would be MS or worse if this exotic pathogen was to establish here (William Cuddy, personal communication). Any samples of stripe rust on barley or barley grass should be submitted to the ACRCP for pathotype determination. The higher probability of summer rainfall in northern NSW/QLD is conducive to the survival of volunteer wheat between cropping seasons which is commonly referred to as the green bridge. When combined with a wide spread in sowing times of roughly between March for dual purpose wheat varieties through to June for quicker maturing main season varieties this situation is quite conducive to the survival and development of stripe rust.
Early and severe infection levels developed at the North Star site, with the WA Yr17+27+ confirmed as the dominant pathotype in the trial. The WA Yr17+ pathotype developed as a mutation of the original WA pathotype, first being
detected in 2006. This pathotype further mutated to develop virulence for the Yr27 gene with the WA Yr17+27+ pathotype first detected in 2011, which reduced the resistance level of varieties such as Livingston and Merinda (Figure 1). All three pathotypes are now distributed across the northern region including into QLD.
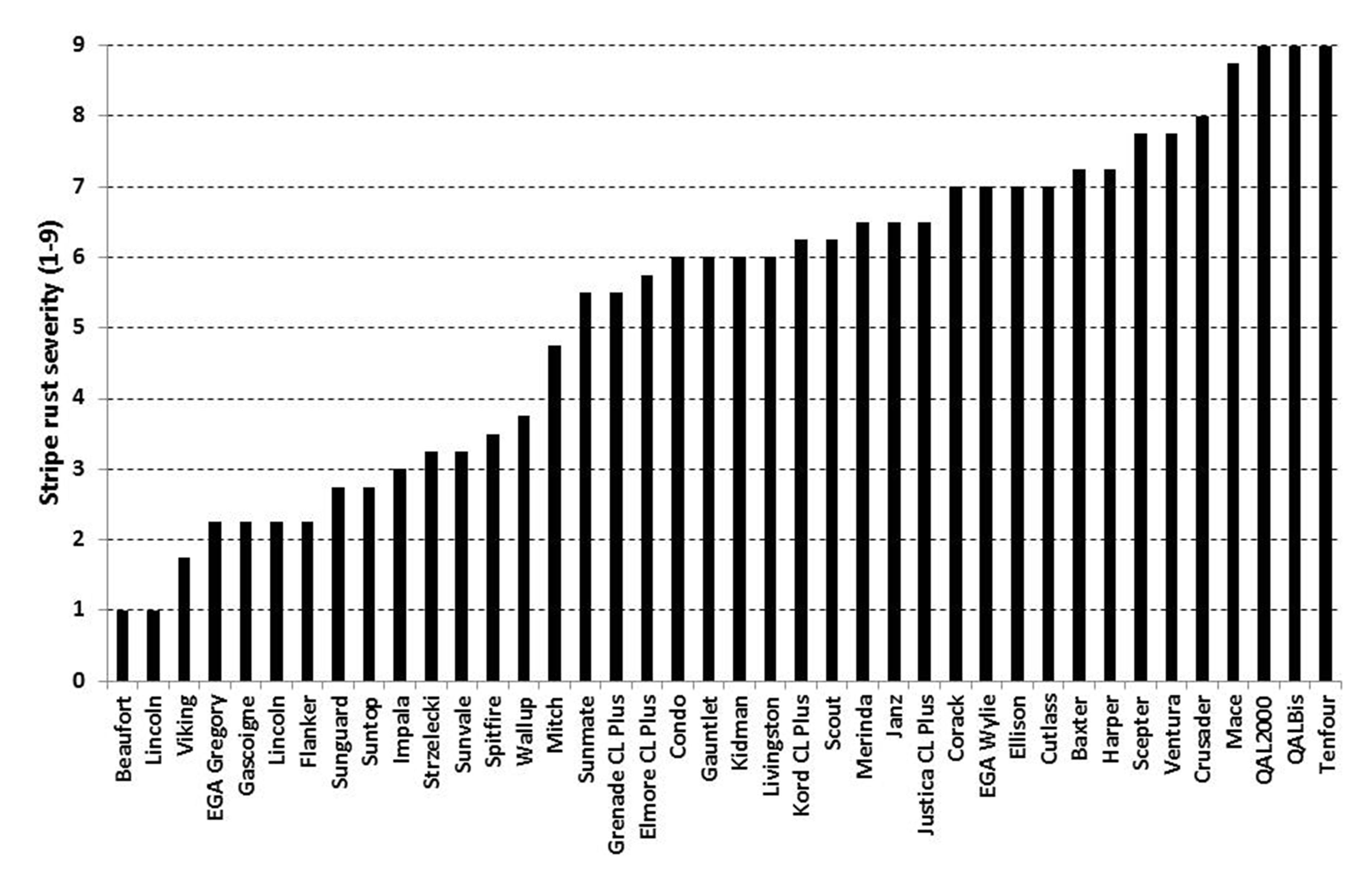
Figure 1. Stripe rust reaction of released wheat varieties in main season NVT - North Star 2015
(Note trial site had early and high stripe rust pressure in 2015 from the WA 17+27+ pathotype. Scores are on the ACRCP 1-9 scale where 1 = no pustules evident and 9 = whole leaf covered in pustules. Individual site data is presented above and is not an overall variety rating, which is derived from reactions in multiple trials across regions and seasons)
Stripe rust in EGA Gregory
Two reports of the supposed 'break down' of stripe rust resistance in EGA Gregory occurred around Wongarbon and Warialda in 2015. NSW DPI inspected the EGA Gregory crop at Wongarbon and there were 'hot individual plants' NOT 'hot-spots' evident in the crop. A ‘hot-spot’ is all plants in at least a 1m circle with development of pustules, and occurs due to higher humidity during winter causing spores to remain in small clumps that are relatively heavy, which limits spread by wind. Spread is therefore mostly over small distances, which results in the appearance of ‘hot-spots’ of infection usually first appearing in late winter to early spring. All plants along multiple 1m sections of rows affected by stripe rust were pulled from the EGA Gregory crop at Wongarbon and separated into individual plants. It then became obvious that there were individual plants along the row infected with stripe rust and others with no visible signs of infection. That is, there were infected individual plants (‘hot individual plants’) but not every plant along a 1m section of row and adjoining rows infected with stripe rust. There were no ‘hot-spots’ evident in the paddock. This process was explained and repeated by the consulting agronomist with the Warialda EGA Gregory crop who similarly concluded that it was ‘hot individual plants’ and clearly not ‘hot-spots’. To complete the picture, individual heads from visually infected and uninfected plants from Wongarbon were collected and sent to the University of Southern Queensland (USQ) for molecular analysis to determine varietal purity. Grain collected from 6 out of 6 plants without visible stripe rust infection were identified as EGA Gregory. In contrast seed collected from 5 of 8 plants with stripe rust infection were identified as NOT being EGA Gregory but all had a similar banding pattern indicating they were all the one contaminant. The actual contaminant variety was not determined but clearly it has increased susceptibility to stripe rust. In both situations the concern around stripe rust appears to be related to more susceptible off-types (contaminants) in the EGA Gregory crops. Pure EGA Gregory remains MR to stripe rust and does not require fungicide management. MR varieties such as EGA Gregory can still develop low levels of stripe rust under high pressure as was evident at North Star in 2015 (Figure 1). However, the level of infection, while visible, does not result in the loss of enough green leaf area to cause significant economic yield loss. If growers are concerned about the levels of stripe rust in their EGA Gregory then they should consider freshening up their seed source to one with a known and higher purity.
Stripe rust ‘hot-spots’ in Suntop
‘Hot-spots’ of stripe rust occurred in two crops around Wellington in 2013, several crops across eastern Australia in 2014 and in numerous crops of Suntop in eastern Australia in 2015. ‘Hot-spots’ of stripe rust first appeared in Suntop crops in northern NSW in early-mid August in 2015. ‘Hot-spots’ in Suntop across seasons has been generally linked with higher nitrogen status within paddocks with some paddocks only developing ‘hot-spots’ in the headlands where double the N rate was applied at sowing. Generally, affected Suntop crops had no up-front fungicide management and did not have a fungicide application around GS30, which commonly occurs commercially in combination with an in-crop herbicide. There is no new pathotype of stripe rust with increased virulence to Suntop, and it has been confirmed from different paddocks that there is currently no underlying issue with seed purity. That is, crops are pure Suntop and, furthermore, true ‘hot-spots’ are evident in affected paddocks and not isolated individually infected scattered plants, which would be more indicative of an issue with seed purity.
Suntop is rated MR-MS to stripe rust, which indicates that it requires one fungicide input (up-front or early between GS30-32) to limit disease development. This message can become complicated as it is often tweaked to the likely timing of epidemic development (significant green-bridge over summer is likely to favour an earlier epidemic), conduciveness of environment (west of Newell Highway is generally drier and hotter which reduces disease pressure) and sowing time (earlier sowing likely to be more favourable to stripe rust and early epidemic development). Generally, varieties such as Suntop (MR-MS), Lancer (MR) and even EGA Gregory (MR) rely largely on Adult Plant Resistance (APR) genes that slow down the rate of disease development. In general, the resistance of the plant increases with plant age and as the temperature rises. The APR gene in EGA Gregory (Yr18) generally appears to express earlier than the gene (Yr31) in Suntop. APR in Suntop appears to be more interactive with lower temperatures and higher N levels which both appear to delay the expression of APR within the leaves. The timing of APR expression remains one of the major issues with stripe rust management in the northern grains region, which differs with variety, sowing time, temperature and even N status. When ‘hot-spots’ occurred in many Suntop crops in 2015 the actual infection became more obvious because APR had expressed and killed off infected cells within the leaf and the surrounding cells. This renders the infection non-viable by denying the stripe rust fungus of living cells to survive in. Yellow flecking of the flag leaf and other leaves on uninfected Suntop plants adjacent to the ‘hot-spots’ indicated the active expression of APR killing spores landing and trying to infect these plants. A close inspection of the oldest leaves (seedling leaves) within the ‘hot-spots’ revealed a mass of old discoloured pustules which highlights that the infection had been present in these patches for a considerable period. Suntop still has a very useful level of resistance to stripe rust and is by no means a ‘sucker’ for stripe rust. This fact is often not easily acknowledged at the grower level when Suntop, for example, may be the most susceptible variety they are currently growing. Hence, the appearance of any infection and the estimation of yield loss (loss of green leaf area) can appear exaggerated without a true susceptible variety to compare infection levels with (Figure 1). Personally, the infection levels observed in ‘hot-spots’ of Suntop in 2015 were consistent with an MR-MS reaction. Varieties such as Suntop which rely on APR as their main source of resistance are worthwhile protecting at early growth stages with seed or fertiliser treatments, or an in-crop fungicide application around GS30-32. This will provide protection until APR is expressed later in the season.
Stripe rust management in 2016
The key messages remain the same:
- Control the green bridge (volunteer wheat) at least four weeks prior to sowing to delay the onset of epidemics.
- Select varieties with improved levels of resistance (MR-MS minimum).
- Ensure variety identification/purity.
- Tailoring fungicide strategies to varietal resistance level, rainfall zone, growth stage and seasonal conditions.
- Monitor crops regularly.
With varieties such as Suntop that rely largely on APR, consider using an in-furrow fungicide to protect early growth. Flutriafol on starter fertiliser has been shown to provide extended protection against stripe rust in the northern region and is a more common component of stripe rust management strategies for susceptible wheat varieties in the southern region. Fluquinconazole seed treatment also protects early growth but tends to not provide the same length of protection as in-furrow treatments in northern trials.
Be ‘alert not alarmed’ for leaf rust pathotypes
There are two new pathotypes of leaf rust of potential significance to northern NSW and QLD. The first (76-3,5,7,9,10,12,13 +Lr37) was a mutation of an existing pathotype with combined virulence for the genes Lr13, Lr24 and Lr37. It was first detected around Warialda on the feed wheat variety Naparoo in 2013 and has only really caused issues in this variety in subsequent seasons. A newer 'SA pathotype' of leaf rust (104-1,3,4,6,7,8,10,12 +Lr37) is an exotic introduction to Australia and was first detected in South Australia in 2014. The SA pathotype of leaf rust has rapidly spread north being detected at Dunedoo, Tamworth, North Star, and Gatton in 2015, but not at levels that warranted fungicide application. However, this is a warning for subsequent years, and growers should check the rating of varieties to these new leaf rust pathotypes with a minimum disease standard of MS recommended by the ACRCP for the northern region. Impala, which has a leaf rust rating of S to existing leaf rust pathotypes, has required fungicide management in northern NSW in recent seasons when conditions (humid and temperatures between 15⁰C to 25⁰C) have been conducive to disease development.
Avoid growing susceptible varieties – has the message changed?
No!
The northern region did the right thing by moving away from growing very susceptible varieties such as H45. Numerous field trials across the northern region, largely GRDC funded NVT trials where stripe rust development is routinely controlled with a fungicide management program, highlight that there is no yield penalty associated with growing newer varieties with improved levels of stripe rust resistance. There is a big difference in the level of fungicide intervention required across a season with a susceptible variety (likely three fungicide inputs) compared to an MR-MS variety (one fungicide ‘up-front’ or around GS30-32) to manage stripe rust in the northern region.
Why are minimum disease standards important?
Minimum disease standards of MR-MS for stripe and stem rust and MS for leaf rust are recommended by the Consultative Committee of the ACRCP for wheat varieties in the northern region (QLD and north NSW). Selecting varieties with these minimum levels of resistance can: reduce the build-up of rust epidemics within the region (the more susceptible the variety the bigger issue they are as a green bridge); decrease disease pressure from existing rust pathotypes within the season; reduce the probability of mutations within existing pathotypes occurring with increased virulence to existing rust-resistance genes; and can reduce the reliance on fungicides as a management tool. The continued production of susceptible and very susceptible varieties, while stripe rust can be controlled with fungicides, jeopardises current and future disease resistance genes. The problem is if you choose to go this way and become reliant on the continuous use of fungicides in susceptible varieties then you are potentially making that decision for the whole industry, as rust spores can be spread large distances by wind. Mutations that develop on your farm rapidly spread across regions.
Is fungicide resistance an issue?
A mini-review was recently written on the risk of rust fungi developing resistance to fungicides (Oliver 2014). To summarise, the rust fungi are classified as having a low risk of developing fungicide resistance, which appears justified by long fungicide usage patterns mainly overseas with no confirmed cases of agronomically significant fungicide resistance being reported in a rust pathogen species. The general conclusions from the review were, "Rust fungi have a reputation that suggests they are immune to the development of fungicide resistance, and this has led growers to rely heavily on fungicides for the control of diseases such as stripe rust. This reputation is based on a long history, during which many other species have developed often disastrous resistance to fungicides, especially Botrytis, Zymoseptoria and powdery mildews, while rusts have remained well controlled.” Within Australia, barley powdery mildew in WA and Septoria tritici blotch (Zymoseptoria, STB) of wheat in the southern region have been reported in recent years to have developed partial resistance to triazoles. In terms of cereal rusts, the strobilurins (Group 11) are, "protected by a serendipitous intron," while, "DMI fungicides (triazoles, Group 3) are protected by relatively low resistance factors and SDHIs (Group 7; medium to high risk of resistance development) have been protected by mixing with other fungicides and their recent introduction.” However, the reviewer still urges vigilance when it comes to the rusts.
It is interesting that in the review (Oliver 2014) the argument that rust fungi are regularly exposed to fungicides but have not yet developed any resistance was largely based on more extended use patterns overseas, “In Europe, where fungicide use on wheat is close to universal, rusts have been regularly exposed to fungicides, even though other species, such as Blumeria graminis (powdery mildew) and Zymoseptoria tritici (STB), are the prime targets." It is therefore not too hard to imagine that the converse could occur in Australia, where reliance on controlling stripe rust in susceptible wheat varieties is the main target for repeat fungicide applications. This practice is potentially selecting for resistance in other fungal species such as powdery mildew and STB that have a medium to high risk for developing fungicide resistance. Fungicide development and the search for new chemistries in Europe are continually driven by the evolution of resistance within STB to existing and recently released fungicides. This is NOT a good scenario and as an industry we would be wise to learn from their mistakes!
Acknowledgements
The research undertaken as part of project DAN00176 is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. The project is co-funded by the Government of NSW through the NSW DPI, who are also thanked for their support in fully funding my position and laboratory and other infrastructure costs. Information on changes in cereal pathotypes and distribution are developed through annual surveys conducted by the Australian Cereal Rust Program which is led by the University of Sydney with funding from GRDC and NSW DPI. Molecular analysis of varietal purity of EGA Gregory seed collected from the crop near Wongarbon was kindly conducted by Dr Anke Martin at the University of Southern Queensland. Stripe rust variety evaluations were conducted under the GRDC funded NVT system with trials in NSW coordinated by Dr Andrew Milgate (NSW DPI, Wagga Wagga).
References
Oliver RP (2014) A reassessment of the risk of rust fungi developing resistance to fungicides. Pest Management Science, 70: 1641-1645.
Further information
Contact details
Steven Simpfendorfer
NSW DPI
Ph: 0439 581 672
Email: steven.simpfendorfer@dpi.nsw.gov.au
Reviewed by
Professor Robert Park, Dr William Cuddy and Dr Guy McMullen
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994GRDC Project Code: DAN00176,
Was this page helpful?
YOUR FEEDBACK
