Herbicide resistance – where we are, where we are going and what can we do about it
Author: Christopher Preston, Peter Boutsalis, David Brunton, Samuel Kleemann and Gurjeet Gill (School of Agriculture, Food & Wine, University of Adelaide) | Date: 13 Feb 2018
Take home messages
- Herbicide resistance occurs most commonly in continuously cropped grain production fields.
- Grass weeds with resistance to pre-emergent herbicides (Groups D and J) and broadleaf weeds with resistance to Group I herbicides are emerging problems that will test growers’ management skills.
- Attempting to introduce three effective control practices in each crop will enable weed seed banks to be reduced.
Where are we with herbicide resistance?
Currently in Australia there are 50 weed species with resistance to herbicides from 11 modes of action. These include 23 grass species resistant to eight modes of action and 27 broadleaf weed species resistant to seven modes of action. Herbicide resistance is known from all six states of Australia, but some states, notably WA, SA and VIC, tend to have more resistance than other states. Herbicide resistance has occurred in all situations where herbicides are used, but is most common and most widespread in grain cropping. Of the 91 weed species by herbicide modes of action with resistance listed in Table 1, 65 have occurred in grain cropping systems. Herbicide resistance in grass species is typically the most problematic issue in grain production due to the dominance of cereals in cropping rotations.
With investment from GRDC, University of Adelaide and Charles Sturt University weed resistance survey data suggests that most, if not all, grain growers have some herbicide resistance on their farms. Resistance is particularly prominent to Group A and B post-emergent herbicides, but is also present to herbicides from Groups C, D, F, I, J, L, M, and Z. Herbicide resistance can also move between locations on wind, in water, in hay and in machinery. However, this only becomes an issue when growers are using the same weed management practices.
Table 1. Herbicide resistant weeds in Australia.
Grass weeds | Groups | States present |
|---|---|---|
Annual ryegrass (Lolium rigidum) | A B C D J L M Q* | WA, SA, VIC, TAS, NSW WA, SA, VIC, TAS, NSW WA, SA, VIC WA, SA, VIC, NSW SA, VIC, NSW WA, SA WA, SA, VIC, NSW WA, SA |
Winter grass (Poa annua) | B* C* D* M* Z* | SA, VIC, NSW SA, VIC, NSW SA, VIC, NSW SA, VIC, NSW SA, NSW |
Barley grass (Hordeum glaucum) | A B L M | SA WA, SA, VIC SA, VIC SA |
Barley grass (Hordeum leporinum) | A B L | SA SA, VIC SA, VIC, TAS, NSW |
Wild oats (Avenafatua) | A B Z | WA, SA, VIC, NSW, QLD SA, VIC, NSW, QLD NSW, QLD |
Wild oats (Avenasterilis) | A B Z | SA, VIC, NSW, QLD SA, VIC, NSW, QLD |
Great brome (Bromus diandrus) | A B M | SA, VIC SA, VIC SA, VIC |
Brome grass (Bromus rigidus) | A B | SA WA, SA |
Red brome (Bromus rubens) | M | WA |
Large crabgrass (Digitaria sanguinalis) | A* B* | SA SA |
Crowsfoot grass (Eluesine indica) | A* L* | QLD QLD |
Paradoxa grass (Phalaris paradoxa) | A B | NSW NSW |
Lesser canary grass (Phalaris minor) | A B | VICVIC |
Liverseed grass (Urochloa panicoides) | C M | QLD NSW |
Silver grass (Vulpia bromoides) | C* L* | WA, VIC VIC |
Awnless barnyard grass (Echinochloa colona) | M | WA, NSW, QLD |
Barnyard grass (Echinochloa crus-galli) | C | NSW |
Annual veldtgrass (Ehrharta longiflora) | A* | WA |
Feathertop Rhodes grass (Chloris virgata) | M | SA, NSW, QLD |
Windmill grass (Chloris truncata) | M | WA, VIC, NSW |
Sweet summer grass (Brachiaria eruciformis) | M | QLD |
Giant Parramatta grass (Sporobolus fertilis) | J* | NSW |
Serrated tussock (Nassella trichotoma) | J* | VIC |
Broadleaf weeds | Groups | States present |
Wild radish (Raphanus raphanistrum) | B C F I M | WA, SA, VIC, NSW, QLD WA WA, SA WA, SA, VIC, NSW WA |
Indian hedge mustard (Sisymbrium orientale) | B C F I | WA, SA, VIC, NSW VIC SA, VIC SA |
African turnip weed (Sisymbrium thellungii) | B | QLD |
Common sowthistle (Sonchus oleraceus) | B I M | SA, VIC, TAS, NSW, Qld SA, VIC, NSW NSW, Qld |
Prickly lettuce (Lactuca serriola) | B M | SA, VIC VIC |
Willow-leaved lettuce (Lactuca saligna) | M | WA |
Capeweed (Arctotheca calendula) | I L* | SA VIC |
Fleabane (Conyza bonariensis) | B M | VIC SA, VIC, NSW, QLD |
Tall fleabane (Conyza sumatrensis) | M* | NSW |
Arrowhead (Sagittaria montevidensis) | B* | NSW |
Black bindweed (Polygonum convolvulus) | B | QLD |
Bedstraw (Galium tricornutum) | B | SA |
Calomba daisy (Pentzia suffruticosa) | B | SA |
Charlock (Sinapis arvensis) | B | NSW |
Dirty Dora (Cyperus difformis) | B* | NSW |
Iceplant (Mesembryanthemum crystallinum) | B | SA |
Lincoln weed (Diplotaxis tenuifolia) | B | SA |
Paterson’s curse (Echium plantagineum) | B* | WA, SA |
Starfruit (Damasonium minus) | B* | NSW |
Turnip weed (Rapistrum rugosum) | B | QLD |
Wild turnip (Brassica tournefortii) | B | WA, SA |
Stinging nettle (Urtica urens) | C* | VIC |
Dense-flowered fumitory (Fumaria densiflora) | D | SA, NSW |
Black nightshade (Solanum nigrum) | L* | QLD |
Pennsylvania cudweed (Gamochaeta pensylvanica) | L* | QLD |
Small square weed (Mitracarpus hirtus) | L* | QLD |
Tridax (Tridax procumbens) | M* | WA |
* This resistance is not known from grain production systems.
Where are we going?
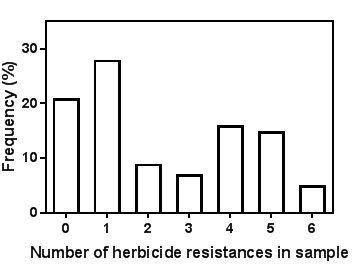
Data from our (University of Adelaide) weed resistance surveys shows that with annual ryegrass, the number of herbicides that any one grower has resistance is bimodal with approx. 40% of fields having resistance to four to six herbicides and about half having resistance to zero or one herbicide (Figure 1). When we looked back at where these fields were located, those with resistance to four to six herbicides were mostly from areas where continuous cropping was common and those with resistance to zero or one herbicide were from areas where pasture was common in rotations. Having pasture in rotations reduces the risk of herbicide resistance, because most growers use few herbicides in the pasture phase. This shows that the major risk factor for developing herbicide resistance is using herbicides. The more focus on continuous cropping and the more herbicides used, the more likely resistance will occur.
Figure 1. Frequency of randomly collected annual ryegrass samples from SA and VIC with resistance to a suite of six common herbicides.
As resistance to post-emergent herbicides in grass weeds, particularly in annual ryegrass, has increased, reliance on pre-emergent herbicides has become more common. This has resulted in increasing resistance to pre-emergent herbicides. There has been resistance to trifluralin in SA for many years, however, the extent of resistance has increased in the past 15 years and it is now common in VIC and increasing in NSW. Resistance to trifluralin became widespread in SA by 2005, resulting in early adoption of Boxer Gold® when it was released in 2008. The heavy dependence on Group J herbicides has led to resistance to this mode of action in the past few years. Resistance to Group J herbicides in annual ryegrass has occurred in SA, VIC and NSW. In all cases, the populations also have resistance to trifluralin, suggesting that once trifluralin has failed, selection pressure shifts to other pre-emergent herbicides.
In one population of annual ryegrass with resistance to Group J herbicides that has been well characterised, resistance occurs across many herbicides of this mode of action (Table 2). This population also has resistance to trifluralin and a reduction in susceptibility to both propyzamide and pyroxasulfone (Sakura®). The current reliance on pre-emergent herbicides for annual ryegrass control could be threatened if more populations like this appear.
Table 2. Concentration of various pre-emergent herbicides required for 50% mortality (LD50) of resistant (R) and susceptible (S) annual ryegrass populations with resistance index (RI).
| Annual ryegrass population | ||||
|---|---|---|---|---|
| Herbicide (with Group) | SLR4 (S) | VLR1 (S) | EP162 (R) | RI* |
| LD50 (g a.i. ha-1) | ||||
Triallate (J) | 248 | 181 | 3188 | 14.9 |
| Prosulfocarb (J) | 311 | 246 | 2608 | 9.4 |
| EPTC (J) | 305 | 288 | 2867 | 9.7 |
| Thiobencarb (J)** | 370 | 265 | 4332 | 13.6 |
| Trifluralin (D) | 39 | 27 | 455 | 13.8 |
| Propyzamide (D) | 30 | 23 | 74 | 2.7 |
| Pyroxasulfone (K) | 9.5 | 6.2 | 64 | 8.1 |
*RI = LD50 of R population divided by LD50 of S populations.
**Thiobencarb is not registered for annual ryegrass control and is shown for comparison purposes only.
Another recent concern is the evolution of resistance to the Group I herbicides in broadleaf weeds. It was once considered to be difficult to develop resistance to Group I herbicides, but the frequency of their use has increased following widespread resistance to Group B herbicides. This has resulted in more important broadleaf weed species with resistance to Group I herbicides (Table 1). These include wild radish, Indian hedge mustard, common sowthistle and capeweed. Perhaps one of the more troubling is common sowthistle, which now has resistance to Group B herbicides, both sulfonylureas (SUs) and imidazolinones (IMIs), glyphosate and all Group I herbicides. This eliminates all the inexpensive options for summer weed control. Given their increasing use in cropping systems, further weeds with resistance to Group I herbicides should be expected.
What can we do about it?
Herbicide management
There are a few strategies involving herbicide management that can be used to reduce herbicide resistance. Rotation of herbicides is one strategy. Rotation of herbicides does not stop resistance from occurring, but can delay resistance. Table 3 is an updated estimate of the number of years of use for different herbicide modes of action. Resistance is more likely to some modes of action than to others, so resistance can be delayed by using high risk modes of action less often.
Table 3. Number of years using a particular herbicide mode of action before herbicide resistance is likely to be a problem.
| Herbicide group | Years of application before resistance is likely |
|---|---|
| A | 6-8 |
| B | 4 |
| C | 10-15 |
| D | 10-15 |
| F | 10 |
| I | >20 |
| J | 6-8 |
| L | >15 |
| M | 12-15 |
Sometimes, resistance does not occur to all herbicides from a mode of action, allowing use of one or more herbicides from that group to control resistant weeds. For example, clethodim controlled many populations of annual ryegrass that were resistant to other Group A herbicides for many years. However, the same rules do not apply to other grass weed species. Likewise, IMI herbicides can be used to control some broadleaf weeds with resistance to other Group B herbicides. For example, until recently there was very little IMI resistance present in common sowthistle, despite resistance to SU herbicides being widespread. A greater dependence on IMI herbicides for weed control, particularly in lentil production, has seen a rapid selection for IMI resistance in this species. As the extent of resistance within a herbicide mode of action can vary from species to species, the only way to be sure is to test for susceptibility in the resistant population.
Do herbicide rates matter?
The answer to this question is yes and no. Both low rates and high rates of herbicides select for resistance in weeds. Typically, high rates will select for resistance faster, because the selection pressure is stronger. However, low rates can select for weak resistance mechanisms and can result in resistant populations with more complex mixtures of resistance mechanisms. Probably of more significance than worrying about herbicide rates is to ensure herbicides are used to provide as effective weed control as possible.
Non-chemical tactics
Non-chemical tactics are, on their own, not as effective or easy to use as herbicides. This is why growers often opt for herbicides as the first control option. However, non-chemical tactics can help reduce the pressure on herbicides and delay resistance. Employing non-chemical tactics is complex and the correct tactic needs to be chosen for the situation. For example, cultivation is likely to be counter-productive in situations where pre-emergent herbicides are relied on for weed control. Cultivation will distribute the weed seeds through the soil, separating them from the herbicide applied on the soil surface.
The inclusion of non-chemical tactics is likely to be most useful where they help to reduce seed set of weeds. Crop competition is one of these tactics. Increased crop competition can be obtained in numerous ways, including by sowing a more competitive crop, sowing a more competitive variety, increasing crop seeding rates, reducing crop row spacings, grading sowing seed for larger seed, and east-west sowing of cereals in some regions. Recent research in the southern region has shown that early sowing of wheat and sowing hybrid canola instead of open-pollinated canola can both reduce annual ryegrass seed production by up to 50%.
Another set of non-chemical tactics is harvest weed seed destruction. These tactics rely on collecting and destroying weed seed that enters the harvester. There are numerous practices that have been used including chaff carts, narrow windrow burning, chaff lining and the Harrington Seed Destructor (HSD). These practices can reduce weed emergence by approximately 50% in the next season. They do rely on a significant amount of the weed seed being captured in the harvest operation and will not be effective on weed seeds that cannot be harvested.
Crop rotations
Choosing the right crop rotation can help introduce tactics for managing troublesome herbicide resistant weeds. Cereal crops are often the best option for managing herbicide resistant broadleaf weeds, due to the range of tactics that can be included. Likewise, break crops are often the best option for managing herbicide resistant grass weeds. Rotations that have too many cereal crops often become infested with herbicide resistant grass weeds. Likewise, rotations with a high intensity of pulse crops will have problems with herbicide resistant broadleaf weeds.
Achieving better weed management
Our long term weed management trials have emphasised the importance of weed seed set reduction in improving long term management of herbicide resistant weeds. Reducing the number of herbicide resistant weed seeds returning to the soil seed bank needs to be a component of any management program for herbicide resistant weeds. It is also important to maintain yields and reduce the number of weeds competing with the crop. Effective use of pre-emergent herbicides has proved useful in managing herbicide resistant annual ryegrass and wild radish.
The other outcome of these long term trials is that achieving three effective control measures in as many crops in the rotation as possible allows weed seed banks to be reduced. For annual ryegrass, this can be pre-emergent herbicides, crop competition and weed seed set control. For wild radish, crop competition and two herbicide applications or two herbicide applications and seed set control have been required.
Useful resources
GRDC Intergrated Weed Management Hub
The University of Adelaide Weed Science publications
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the authors would like to thank them for their continued support.
Contact details
Christopher Preston
University of Adelaide
08 8313 7237
christopher.preston@adelaide.edu.au
GRDC Project Code: UA00159, UCS00020, USC00020, UCS00024, US00084,
Was this page helpful?
YOUR FEEDBACK