Impacts of residual herbicides on soil biological function
Take home messages
- Numerous factors (such as herbicide group, application rate and frequency, soil type, crop type and environmental conditions) influence the expression of herbicide persistence in soil. Results to date imply that risk to crop growth through direct phytotoxicity (plant-back) is more important than through impacts on soil biological processes.
- Herbicides with longer residual lives such as imazapic, products that contain picloram, and diuron can potentially significantly reduce chickpea shoot and root growth as well as number and dry weight of the nodules of chickpea plants when applied 90 days prior to planting. Nitrogen fixation would therefore be impacted, leading to potential N deficiency in the pulse and of the following crop. Careful adherence to recommended plant back periods for sensitive crops is critical.
- Mycorrhizal colonisation of crops does not appear to be negatively impacted by herbicide residues in soils unless major root damage has occurred.
Background
Over the last thirty years, the number of Australian grain growers using reduced-till technology has increased from around 5% to over 70%. This has dramatically improved soil structure and reduced erosion, but has also necessitated an increase in herbicide use for weed control. Little is known about how increased herbicide use might impact on soil organisms and the beneficial functions they provide. Anecdotal evidence also suggests that plant-back damage in rotational crops due to herbicide residues is a growing concern amongst growers, but the scale and cost to the Australian Grains industry remains unknown. A GRDC-funded project (DAN00180) has been benchmarking the level of herbicide residues in cropping soils and generating new knowledge about the fate, behaviour and risk of herbicides to productivity and soil biological function. The aim is to enable the Australian grains industry to better understand the risks and implement changes in management for more productive and resilient farming systems.
One part of this project has been examining the impacts of residual herbicides applied over a summer fallow on subsequent biological symbiotic associations (rhizobia and mycorrhizal) in winter crops (chickpea and wheat) and biological components (nematode communities, microbial activity) of the soil. Field trials conducted by the Regional Research Agronomy network and the weeds teams within the Sustainable Farming Systems group of Crop and Food Science, Qld DAF, have given an opportunity to viably assess the impact of some residual herbicides on certain crop associated soil biological functions and components. With the increase in glyphosate resistance and difficult to control weeds in the northern region, alternative weed management tactics are required. One such alternative is residual herbicides. Residual herbicides can provide medium to long-term control of weeds in fallow and crop by controlling several flushes of emergence. However the efficacy of residual herbicides can be affected by the environment (soil type, rainfall, temperature). Therefore, it is necessary to gather local efficacy and persistence data across a range of environments and seasons. Persistence of residual herbicides can have flow-on effects on the biological components of soils either directly or indirectly (through reduced plant and root growth).
What was done
Residual herbicide trials were conducted across Queensland over the summers of 2015/16 and 2016/17. Multiple sites operated out of Emerald, Goondiwindi and Toowoomba (15 sites in total) were established to investigate the efficacy of residual herbicides on key summer weeds. Bioassays using soil samples collected 90 days post-spraying were then conducted to study the impact on soil biota such as mycorrhizal fungi and beneficial nematodes as soil health indicators and on key biological associations such as nodulation with N fixing bacteria.
Site
Each field trial was established at a site/s where there was an expected uniform density of a minimum 30-50 plants/m2 for each flush of emergence (often difficult to predict). This was to ensure there would be enough target weed species to distinguish between treatments either alone or in mixture.
Experimental design
- Randomised block
- 3 replications x 16 herbicide treatments (Table 1)
- Pot size: 8m x 2m
Table 1. Treatments (note not all treatments applied at all sites)
Trt No. | MOA | Product/s | Rate (/ha) | Active ingredient, grams active/L(kg) | g.a.i./ha |
|---|---|---|---|---|---|
1 | - | Untreated control | - | ||
2 | B | Flame® | 200 mL | Imazapic 240 gai/L | 48 |
3 | D | Stomp® Xtra** | 3.3 L | Pendimethalin 455 gai/L | 1500 |
4 | C | Terbyne® | 1.4 kg | Terbuthylazine 750 gai/kg | 1050 |
5 | H | Balance® | 100 g | Isoxaflutole 750 gai/kg | 75 |
6 | K | Dual Gold® | 2 L | S-metolachlor 960 gai/L | 1920 |
7 | B + H | Flame + Balance | 200 mL + 100 g | Imazapic 240gai/L + | 48 75 |
8 | B + K | Flame + Dual Gold | 200 mL + 2L | Imazapic 240gai/L + | 48 1920 |
9 | B + D | Flame + Stomp Xtra | 200 mL + 3.3 L | Imazapic 240gai/L + | 48 1500 |
10 | D + H | Stomp Xtra + Balance | 3.3 L + 100 g | Pendimethalin 455gai/L + | 1500 75 |
11 | C | Nu-trazineTM** | 1 kg | Atrazine 900gai/kg | 900 |
12 | C | Diuron 900 DF | 1 kg | Diuron 900gai/kg | 900 |
13 | G | Sharpen® | 34 g | Saflufenacil 700gai/kg | 24 |
14 | I | FallowBossTM TordonTM | 1 L | 2,4-D amine at 300gai/L + | 300 75 7.5 |
15 | K | Outlook®# | 1 L | Dimethenamid-P 720gai/L | 720 |
16 | D | TriflurX®# | 3 L | Trifluralin 480gai/L | 1440 |
** Please note that Stomp Xtra and Nu-trazine were used as a treatment in this trial and are no longer registered products, however other products are available containing the same active ingredients are registered. Always refer to label for rates.
# Products not registered in fallow.
Application of treatments
- To start with a weed-free trial site, weeds were initially sprayed out with a non-residual knockdown herbicide (eg. glyphosate or paraquat).
- Herbicide treatments were applied at a spray volume of 100 L/ha using 015 even flat fan nozzles on a 2m shielded boom.
- At most sites, herbicides were incorporated by rain.
Bioassays
A minimum of 5 kg of soil to a depth of 10cm from each plot was collected 90 days post application. Soil from each treatment at each site was potted into 3 x 1.5 L pots and planted to either uninoculated chickpea or inoculated (with rhizobia, strain CC1192) chickpea or to wheat. Soil was also collected for a nematode community analysis at time of sampling and for soil physical characteristics. Whilst an analysis of the level of herbicide residue at this time would also be of interest, it was cost prohibitive to do all samples and so specific plots/treatments only will be analysed.
Plants were grown for 8 weeks, at which time top and root growth was assessed and nodulation due to rhizobia was scored for each of the chickpea treatments. Wheat roots were subsampled for mycorrhizal colonisation assessments.
Results
Whilst analyses are still underway, preliminary results are indicating that residual effects of imazapic (Trt 2) and the combination of imazapic and isoxaflutole(Trt 7), of diuron (Trt 12) and of picloram, 2,4-D and aminopyralid (applied as Fallow Boss Tordon) (Trt 14) have all had negative impacts on nodulation and in some cases growth of the plant. Figures 1 and 2 are examples of the impacts on nodulation and plant growth respectively at one site (Denver) in southern Qld. These effects are not surprising, as these active ingredients are all listed as being slowly degraded by microbes with average half-lives of 89 (diuron) to 232 (imazapic) days (Congreve and Cameron 2014). Furthermore plant back periods for chickpea as specified on some key product labels are 4 months for imazapic and 6 months for FallowBoss Tordon (picloram, 2,4-D and aminopyralid). Soil organic matter and the amount and frequency of rainfall will greatly influence degradation times as breakdown is primarily via microbial degradation processes. Temperature will also have an influence, as can incorporation by rainfall or tillage. In the case of imazapic, soil pH will also have an influence, soil half-life values being somewhat longer in lower pH soils. We sampled at 90 days post application, so given the dry season over the summer, several of these slower to degrade herbicides are obviously still persisting with the potential to exhibit negative impacts on plant growth and nodulation of the sensitive species chickpea.
Mycorrhizal colonisation of wheat roots completed for the Denver site have shown no impacts of the herbicides on % colonisation by arbuscular mycorrhizal fungi (AMF), with levels consistently around 50 -60%. Damaged chickpea roots however do have lower mycorrhizal colonisation levels and hence much lower lengths of mycorrhizal root. Nematode communities are also under analysis to determine impacts on soil food web structures.
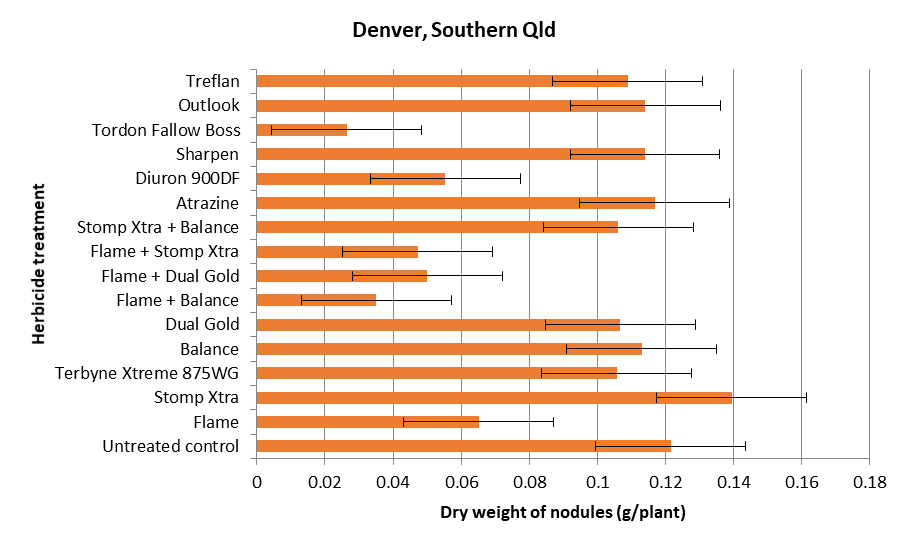
Figure 1. The dry weight of nodules on 8 week-old chickpea plants grown in pots of a vertosol that was collected from a paddock in southern Qld 90 days post application of various residual herbicides.
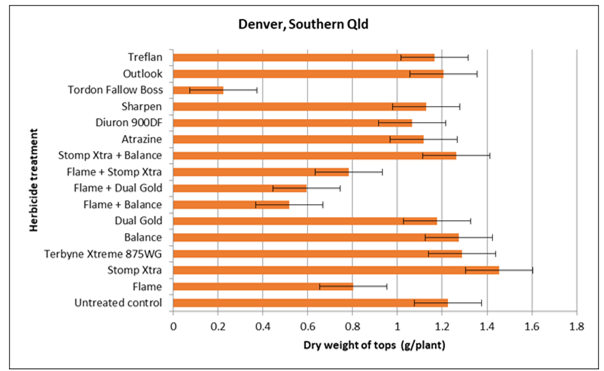
Figure 2. The dry weight of shoots of 8 week-old chickpea plants grown in pots of a vertosol that was collected from a paddock in southern Qld 90 days post application of various residual herbicides
Implications for growers
Growers should carefully adhere to recommended plant back periods for sensitive crops and be especially careful if the seasonal weather conditions have not been conducive to complete herbicide breakdown. Not only will growth of the crops be reduced but damage due to the presence of residual herbicide in the soil may also lead to reduced nodulation and hence reduced nitrogen fixation in a following chickpea crop leading to potential N deficiency in the pulse and of the following cereal crop. Severe root and shoot damage leading to poor crop performance has the potential to reduce mycorrhizal colonisation in following crops.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. Many thanks especially to the DAF Regional Research Agronomy Network team especially Andrew Erbacher, Darren Aisthorpe and Duncan Weir for conducting the trials along with Michael Widderick and his weeds research team.
Contact details
Nikki Seymour
Department of Agriculture and Fisheries
Leslie Research Facility, 13 Holberton St
Toowoomba 4350
Ph: 07 45291237
Fx: 07 46398800
Email: nikki.seymour@daf.qld.gov.au
® Registered trademark
GRDC Project Code: DAN00180,
Was this page helpful?
YOUR FEEDBACK
