Root traits for yield improvement in barley
Author: Hannah Robinson (QAAFI/UQ), Vesna Kandic (Maize Research Institute, Serbia), Jack Christopher (QAAFI/UQ), Glen Fox (QAAFI/Department of Food Science, Stellenbosch University), Alison Kelly (DAF/QAAFI), Andrew Borrell (QAAFI), Lee Hickey (QAAFI/UQ) | Date: 28 Feb 2018
Take home messages
- Seminal root angle is a good indicator of the mature root system architecture in barley.
- There is variation for seminal root angle and seminal root number in commercial barley varieties.
- Pre-breeding barley lines have more extreme measures for seminal root angle (both narrow and wide) and root number (both low and high).
- There is a weak to moderate relationship between seminal root traits and yield improvement in barley, where the direction and magnitude of the correlation is highly environment dependent.
Call to action
- Barley improvement programs (breeding and pre-breeding) need to better understand and utilise the extent of genetic diversity in root angle and root number to enhance productivity in water-limited and non-limited environments.
- Since the relationship between seminal root traits and yield improvement in barley is highly dependent on the environment, it is critical for barley improvement programs to characterise the environment, and to optimise specific adaptation (genotype x management) for a range of target environments.
- Root ideotypes need to be designed for water and nutrient uptake, since nutrient and water reserves can be spatially separated in the soil.
Background
Roots play a vital role in resource uptake and plant growth regulation by being the primary interface for water and nutrient capture. In addition, roots provide anchorage and interact with cooperative organisms in the soil. Defined as the spatial distribution of roots throughout the soil space, root system architecture (RSA) is a complex trait with many underlying processes, such as root elongation, curving and branching (Lynch 1995; Rich and Watt 2013). Furthermore, the RSA of a plant has been shown to influence the efficiency and timing of water capture and extraction in cereal crops (Kondo et al. 2000; Pennisi 2008).
The fibrous root system of cereals is broadly divided into seminal roots, emerging from the primordia in the embryo of the seed, and nodal or secondary roots, developing from the lower nodal regions of the culm throughout tillering (Forster et al. 2007). The growth angle between the first pair of emerging seminal roots, described as the seminal root angle, was found to be representative of the mature root system architecture in wheat (Oyanagi et al. 1993; Manschadi et al. 2008; Manschadi et al. 2010). As a result, seminal root angle is considered a proxy trait for mature RSA in wheat (Sanguineti et al. 2007; Hamada et al. 2012; Christopher et al. 2013; Richard et al. 2015).
Water and nitrate, the two resources most highly acquired by crops, are extremely mobile, leaching into the deeper layers of the soil and reducing availability in the surface strata. Generally, higher levels of water and nitrate are located deeper in the soil profile. These levels are further exaggerated throughout the season under terminal drought conditions, where the soil dries progressively from the surface layers as a result of evaporation, drainage and root uptake.
Environment modelling of the northern grain growing region of Australia identified terminal drought stress as the most common pattern of water-stress for this growing area (Chenu et al. 2011). A deep root system is thought to be optimal for maximum resource capture under water-limited conditions for a number of crop species (Herder et al. 2010; Lynch 2011). Root foraging for resource acquisition is a high metabolic cost for crops (Lynch 2015), thus plants with deep roots in close proximity to resources minimise the need for extensive foraging (Lynch 2013). In addition, plants with the deep roots are also believed to adequately access water and nutrients from the top layers of the soil through their shallow lateral roots (Lynch 2013). Thus, theoretically a narrow yet deep root system would be optimal for cereal crops grown across the northern grain growing region of Australia, where in-season rainfall is limited and terminal drought stress is common. In support of this, a narrow root angle, representative of a steep and deep RSA, improves deep-soil foraging and water extraction under terminal drought in wheat, sorghum and rice (Uga et al. 2011; Manschadi et al. 2008; Mace et al. 2012; Uga et al. 2013). In barley, the value of a narrow, yet deep, RSA for water capture and yield improvement has yet to be completely explored.
In this paper, we begin by investigating seminal root angle as a proxy trait for mature RSA in barley. Next, we explore the variation for seminal root angle and root number in a collection of commercial malt and feed barley varieties. Finally, we take a first look at the relationship between seminal root traits and yield in barley grown in the northern region.
Materials and methods
Phenotyping root traits
To investigate seminal root angle as a proxy trait for mature RSA, a panel of five pre-breeding barley lines were phenotyped for seminal root angle using the ‘clear pot’ method under controlled conditions using a randomised complete block design (Richard et al. 2015). Seminal root angle was defined as the angle measured between the first pair of emerging seminal roots (Figure 1). The ‘clear pot’ method was also used to phenotype seminal root angle and root number in a collection of commercial barley varieties to explore phenotypic variation. The commercial varieties included Commander, Oxford, Fathom, Westminster, Compass, Shepherd, ScopeCL, Baudin, Rosalind and La Trobe.
To examine the relationship between seminal root traits and yield, a panel of 165 pre-breeding lines were also phenotyped for seminal root angle and root number using the ‘clear pot’ method. The panel of pre-breeding lines consisted of five families derived from crosses between Commander and four elite breeding lines from the northern region barley (NRB) breeding program (Warwick, Australia) and one line from the ND24260 × Flagship doubled haploid population (Hickey et al. 2011). To calculate best linear unbiased predictors (BLUPs) for seminal root traits of varieties in all experiments, a linear mixed model was fitted to the data using ASReml-R (Butler et al. 2008). The experimental variation was accounted for by including all design terms as well as spatial location of the pot in each glasshouse experiment.
The mature root system architecture of the five pre-breeding lines was phenotyped in the field at The University of Queensland (UQ) research station, Gatton, Queensland, Australia using the ‘shovelomics’ approach (Trachsel et al. 2011). Mature root system architecture was defined as the outer angle capturing the overall direction of root growth.
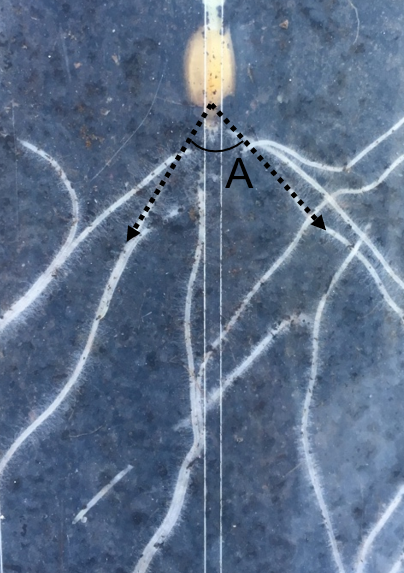
Figure 1. Illustration of seminal root angle measurement of the first pair of seminal roots, where angle (A)
is the measurable seminal root angle.
Yield trials
The panel of 165 pre-breeding lines were evaluated in three yield trials conducted at the Department of Agriculture and Fisheries (DAF) Hermitage research facility (Hermitage, QLD) across 2016 and 2017. Trial environments are detailed in Table 1, where weather data was accessed online from the Bureau of Meteorology (http://www.bom.gov.au/). All field trials were designed as partially replicated (0.5) row-column designs (Cullis et al. 2006). Weeds and diseases were controlled as required. To estimate the association between seminal root traits and yield across the three trials, multi-environment trial (MET) analysis was performed using a linear mixed model and fitting a factor analytic structure for the genetic by environment by trait effects in the model (Smith et al. 2001). Site specific BLUPs for yield and root traits were generated for each pre-breeding line from the model, along with correlations between yield and seminal root traits.
Table 1. Description of three yield trials evaluating panel of 165 pre-breeding lines
Trial | Year | Location | Irrigated | Sown | ARFᵃ (mm) | CRFᵇ (mm) | Mean yield (t/ha) |
|---|---|---|---|---|---|---|---|
H-16-dry | 2016 | Hermitage, QLD, 28.2° S, 152.1° E | No | 22.07.2016 | 729.4 | 370.0 | 5.03 |
H-16-irri | 2016 | Hermitage. QLD, 28.2° S, 152.1° E | Yes | 22.07.2016 | 729.4 | 370.0 | 4.58 |
H-17-dry | 2017 | Hermitage. QLD, 28.2° S, 152.1° E | No | 27.06.2017 | 575.0 | 171.0 | 4.96 |
a Annual rainfall
b Cropping season rainfall
Results
Seminal root angle as a proxy trait for mature RSA
Wide variation for seminal root angle was observed in the panel of pre-breeding lines, where the narrowest angle of 52.0° was observed for pre-breeding line 1 (PB1) and the widest angle of 77.4° for PB2 (Figure 2A). Similar results were identified for mature root angle, where pre-breeding lines were ranked in a similar order to that for seminal root angle (Figure 2B, C). The exception to this was PB2, which displayed a wider seminal root angle compared to mature root angle (Figure 2A, B). Overall, like in wheat, seminal root angle appears to be a satisfactory approximation of the mature RSA in barley. It is important to note that only a small number of lines were examined in this experiment and further investigation using a larger collection of lines is required to validate this result.
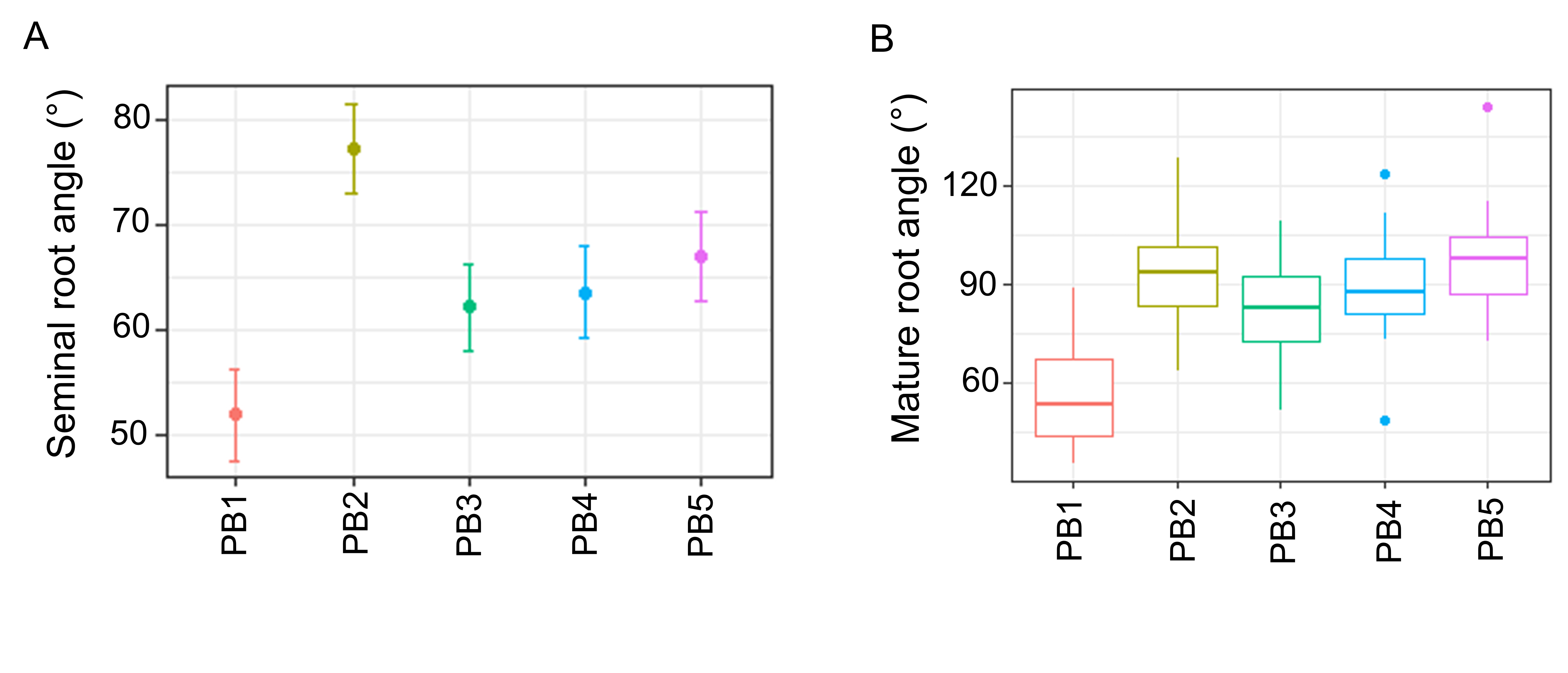
Figure 2. Seminal and mature root angle in the panel of five pre-breeding lines. (A) Seminal root angle BLUPs measured using the ‘clear pot’ method, where error bars represent the standard error of the mean. (B) Box-and-whisker plots displaying the full distribution of data for mature root angle measured using the ‘shovelomics’ methods. Adapted from Voss-Fels and Robinson et al. 2017.
Seminal root trait variation in commercial barley varieties
Seminal root angle and seminal root number varied across the barley varieties (Figure 3A, B). ScopeCL displayed the narrowest root angle (44.1°) and Westminster displayed the widest (64.8°). For root number, ScopeCL displayed the highest number (5.3), whereas the lowest number of roots (4.7) was observed for Commander.
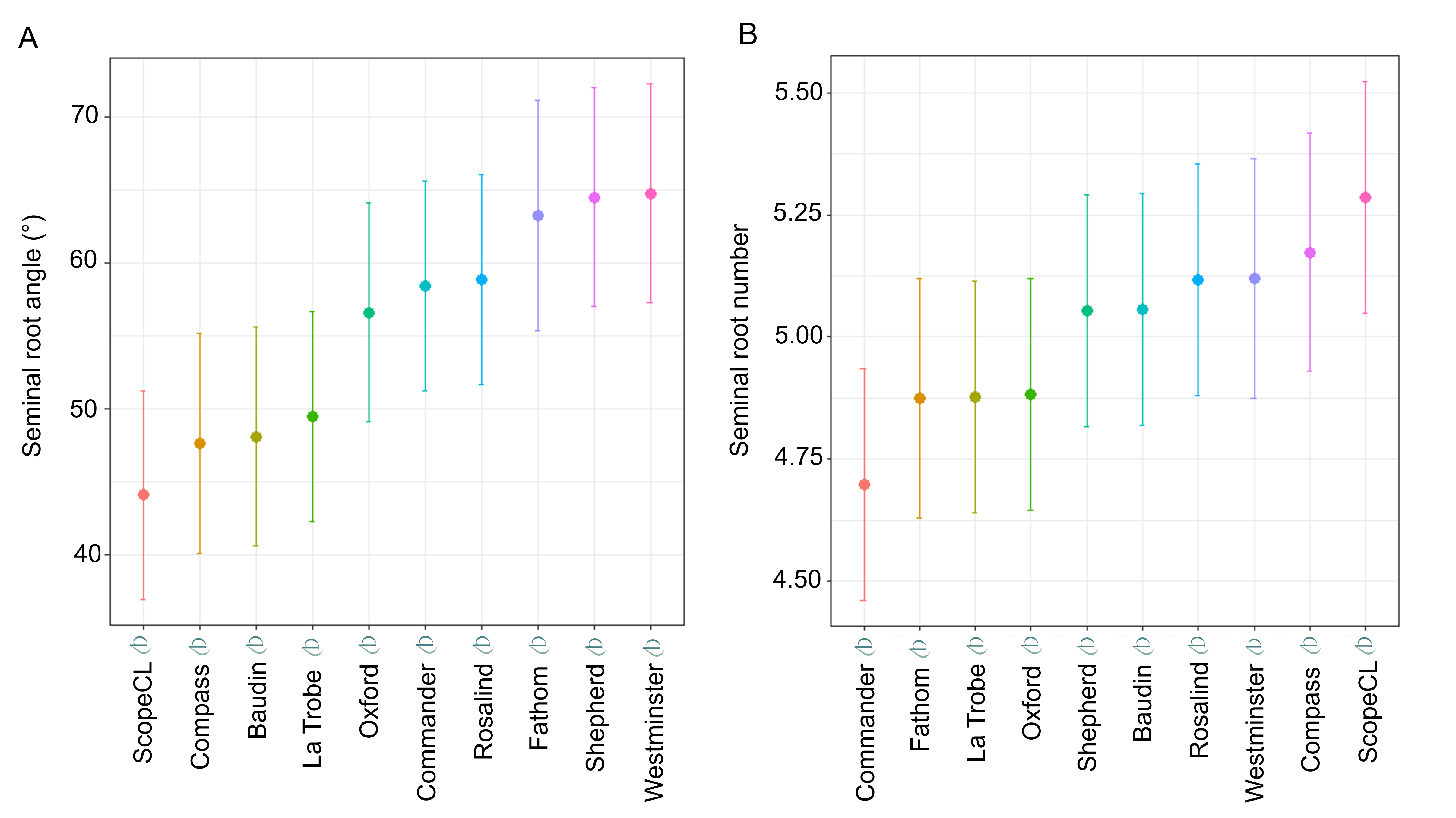
Figure 3. Seminal root trait BLUPs for the collection of 10 commercial barley varieties (A) Seminal root angle and (B) seminal root number BLUPs. Error bars represent standard error of the mean.
Relationship between seminal root traits and yield
A wide range in root phenotypes were observed in the panel of 165 pre-breeding lines, with angle ranging from 12.50° to 109.70° and root number from 3.00 to 6.19 roots (Figure 4A, B). In comparison to the commercial varieties, the pre-breeding lines have a significantly wider range of phenotypes for both seminal root traits. Depending on the value of root traits for yield improvement, these pre-breeding lines may be a useful source of germplasm for introgression of more extreme root phenotypes into breeding material.
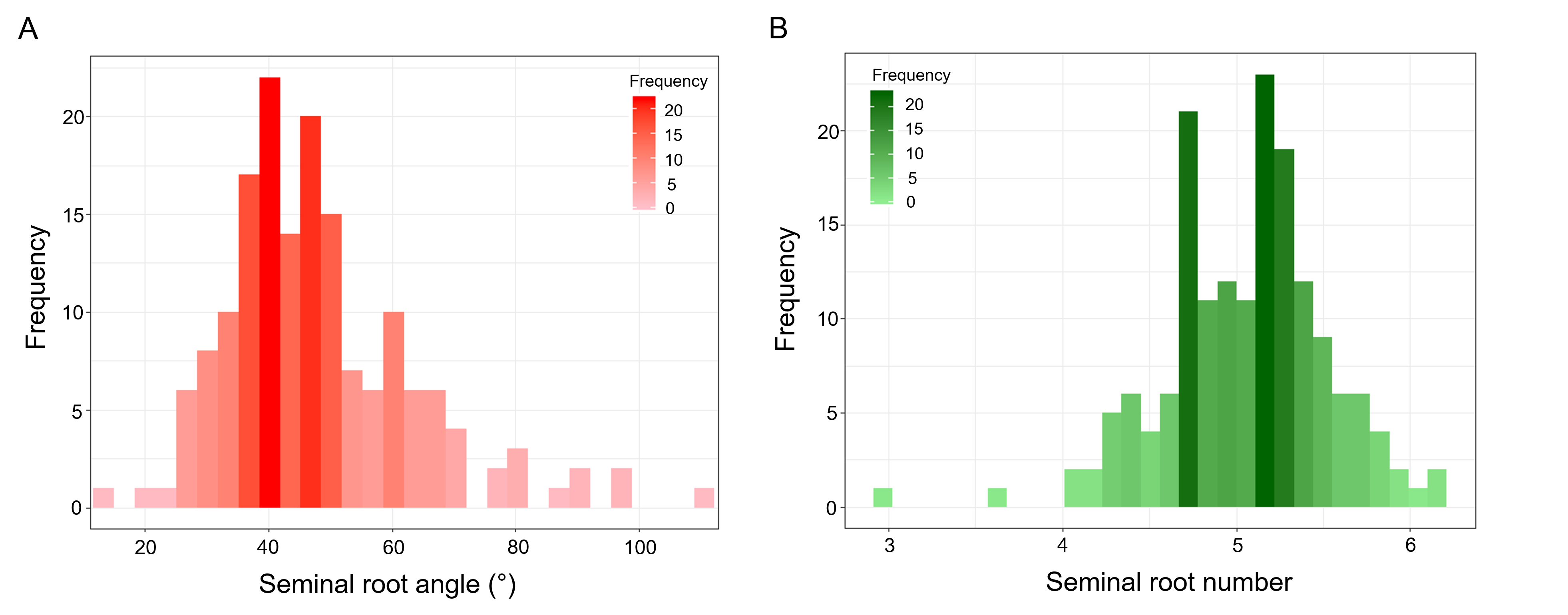
Figure 4. Distributions of root traits for the panel of 165 pre-breeding lines. (A) distribution of seminal root angle (°), and (B) distribution of root number. Increase in darkness represents an increase in frequency of lines with the root phenotype.
Due to uncharacteristically high in-season rainfall throughout the 2016 cropping season (Table 1), the H-16-dry trial was correlated (0.56) with the H-16-irri trial in the MET yield analysis (Figure 5) and was therefore similar in yield to an environment with frequent in-season rainfall. H-17-dry had the lowest in-season rainfall (Table 1) making it the most representative dryland trial of the three trials described in this study. Figure 5 demonstrates there is an association between both seminal root traits and yield, however it appears to be highly environment dependent. In our driest trial, H-17-dry, improved yield was associated with a narrow root angle (-0.28) and a high root number (0.44). In contrast, in the trials characterised by frequent in-season rainfall, a wide root angle with a low root number was associated with increased yield (Figure 5B). Consistent with the current literature, our results suggest a narrow root angle with a high root number may be more beneficial for yield improvement in water-limited environments.
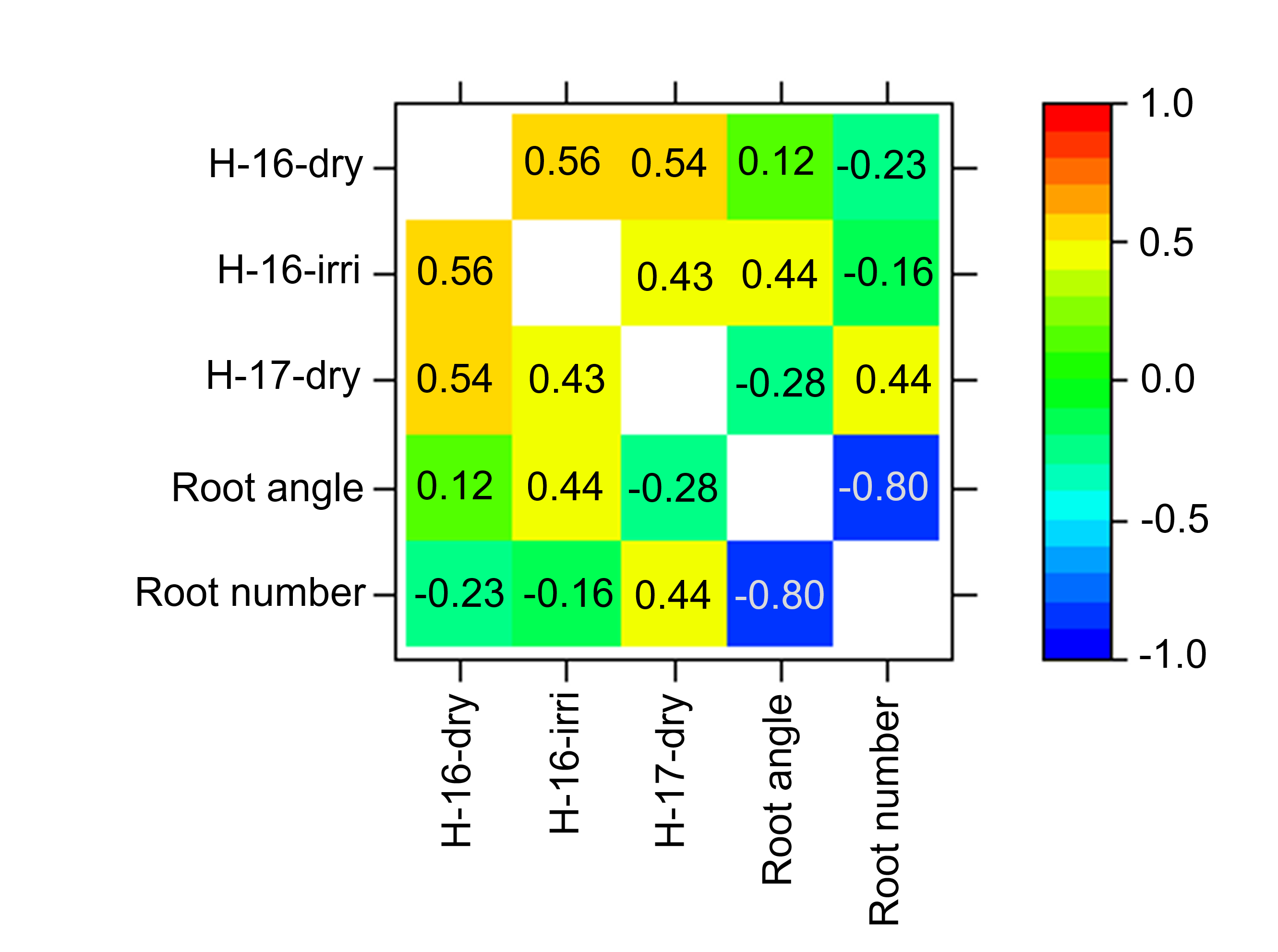
Figure 5. Multi-environment trial analysis for yield and seminal root traits. Heatmap of correlations between yield trials, seminal root angle and seminal root number. Positive correlations between traits increase with increasing colour intensisty (darker) and negative correlations with decreasing colour intensity (paler).
Conclusion
Here, we provide preliminary evidence for seminal root angle as a proxy trait for mature RSA in barley. This result provides support for the use of high-throughput methods to measure seminal root angle to better understand the barley root system and its relationship with yield. Further, using the ‘clear pot’ method to measure seminal root angle and root number, we demonstrated that the collection of commercial barley varieties was wide-ranging for seminal root traits. However, the panel of 165 pre-breeding lines displayed the most extreme measurements for both seminal root traits. This highlights an opportunity to exploit more diverse root traits in commercial breeding material.
Our results reveal that seminal root angle and root number were weakly to moderately associated with yield improvement, where the direction and the magnitude of this relationship was highly environment dependent. For instance, a narrow root angle with a high root number appeared beneficial in drier growing environments where the crop is sown into deep soil with a full moisture profile. This outcome is consistent with the current literature in other cereal crops, where a narrow root system has an increased proportion of roots at depth and thus an improved ability to uptake moisture from deep in the soil profile (Manschadi et al. 2006; Lynch et al. 2014).
In contrast, a wide root angle with a lower root number appeared to be more beneficial for environments that experience frequent in-season rainfall in our study. This is likely because a wide root angle tends to promote shallow root growth, thus increasing the proportion of roots in the top soil strata that can take advantage of in-season rainfall. Despite the consistency of these results with previous research, further investigation in a larger number of environments and across more growing seasons is required to extensively validate the relationship between seminal root traits and yield improvement for barley in the northern grain growing region. In addition, the role of roots in nutrient uptake needs to be better understood, particularly in relation to immobile elements like phosphorus (P) and potassium (K). As nutrient and water reserves can be spatially separated in the soil, root systems will be required to respond to this spatial separation.
References
Butler D, Cullis BR, Gilmour A, Gogel B (2008) ASReml-R Reference Manual. Technical Report 3. Qld Department of Agriculture, Fisheries and Forestry, Brisbane, Australia.
Chenu K, Cooper M, Hammer GL, Mathews KL, Dreccer MF, Chapman SC (2011) Environment characterization as an aid to wheat improvement: interpreting genotype–environment interactions by modelling water-deficit patterns in North-Eastern Australia. J Exp Bot 62:1743-1755
Christopher J, Christopher M, Jennings R, Jones S, Fletcher S, Borrell A, Manschadi A, Jordan D, Mace E, Hammer G (2013) QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theor Appl Genet 126: 1563-1574
Cullis BR, Smith AB, Coombes NE (2006) On the design of early generation variety trials with correlated data. J Agric Biol Environ Stats 11:381
Forster BP, Franckowiak JD, Lundqvist U, Lyon J, Pitkethly I, Thomas WTB (2007) The barley phytomer. Ann Bot 100:725-733
Hickey LT, Lawson W, Platz GJ, Dieters M, Arief VN, German S, Fletcher S, Park RF, Singh D, Pereyra S, Franckowiak J (2011) Mapping Rph20: a gene conferring adult plant resistance to Puccinia hordei in barley. Theor Appl Genet 123:55-68
Hamada, A, Nitta M, Nasuda S, Kato K, Fujita M, Matsunaka H, Okumoto Y (2012) Novel QTLs for growth angle of seminal roots in wheat (Triticum aestivum L.). Plant Soil 354:395-405
Herder GD, Van Isterdael G, Beeckman T, De Smet I (2010) The roots of a new green revolution. Trends Plant Sci 15:600-607
Kondo M, Murty MVR, Aragones DV (2000) Characteristics of root growth and water uptake from soil in upland rice and maize under water stress. Soil Sci Plant Nutr 46:721-732
Lynch J (1995) Root architecture and plant productivity. Plant Physiol 109:7-13
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041-1049
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347-357
Lynch JP (2015) Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant Cell Environ 38:1775-1784
Lynch JP, Chimungu JG, Brown KM (2014) Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J Exp Bot 65:6155-6166
Manschadi AM, Christopher J, Devoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Func Plant Biol 33:823-837
Manschadi AM, Christopher JT, Hammer GL, Devoil P (2010) Experimental and modelling studies of drought‐adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosyst 144:458-462
Manschadi AM, Hammer GL, Christopher JT, deVoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115-129
Oyanagi A, Nakamoto T, Wada M (1993) Relationship between root growth angle of seedlings and vertical distribution of roots in the field in wheat cultivars. Jpn J Crop Sci 62:565-570
Pennisi E (2008) Plant genetics: The blue revolution, drop by drop, gene by gene. Sci 320:171-173
Rich SM, Watt M (2013) Soil conditions and cereal root system architecture: review and considerations for linking Darwin and Weaver. J Exp Bot 64:1193-1208
Richard C, Hickey L, Fletcher S, Jennings R, Chenu K, Christopher J (2015) High-throughput phenotyping of seminal root traits in wheat. Plant Methods 11:13
Sanguineti MC, Li S, Maccaferri M, Corneti S, Rotondo F, Chiari T, Tuberosa R (2007) Genetic dissection of seminal root architecture in elite durum wheat germplasm. Ann Appl Biol 151:291-305
Smith A, Cullis B, Thompson R (2001) Analyzing variety by environment data using multiplicative mixed models and adjustments for spatial field trend. Biometrics 57:1138-1147
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341:75-87
Uga Y, Okuno K, Yano M (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J Exp Bot 62:2485-2494.
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H, Takehisa H, Motoyama R, Nagamura Y, Wu J, Matsumoto T, Takai T, Okuno K, Yano M (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45:1097-1102
Voss-Fels KP*, Robinson H*, Mudge SR, Richard C, Newman S, Wittkop B, Stahl A, Friedt W, Frisch M, Gubur I, Miller-Cooper A, Campbell BC, Kelly A, Fox G, Christopher J, Christopher M, Chenu K, Franckowiak J, Mace ES, Borrell AK, Eagles H, Jordan DR, Botella JR, Hammer G, Godwin ID, Trevaskis B, Snowdon RJ, Hickey LT (2017) VERNALIZATION1 modulates root system architecture in wheat and barley. Mol Plant 0.1016/j.molp.2017.10.005
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. The authors would also like to thank the field staff of the Department of Agriculture and Fisheries at Leslie Research Facility and Hermitage Research Facility for assisting with field trials conducted as part of this study.
Contact details
Hannah Robinson
Queensland Alliance for Agriculture and Food Innovation
The University of Queensland, Brisbane QLD 4072
Ph: 0488 279 694
Email: h.robinson1@uq.edu.au
Dr Lee Hickey
Queensland Alliance for Agriculture and Food Innovation
Room S603, Level 6, Hartley Teakle Building,
The University of Queensland,
Brisbane QLD 4072
Ph: 07 3365 4805
Mb: 0408210286
Email: l.hickey@uq.edu.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: GRS10940,
Was this page helpful?
YOUR FEEDBACK
