Why adhering to integrated Ascochyta rabiei management strategy is now more important than ever to sustain a profitable chickpea industry
Author: Rebecca Ford (Griffith Uni), Kevin Moore (NSW DPI), Prabhakaran Sambasivan (Griffith Uni), Yasir Mehmood (Griffith Uni), Kristy Hobson (NSW DPI), Christine Walela (SARDI Clare), Jason Brand (DEDJT Horsham and Jenny Davidson (SARDI Urrbrae) | Date: 07 Mar 2018
Call to action/take home message
Use the prescribed best practice of rotation and fungicide regimes to minimise the selection pressure and build-up of highly aggressive fungal isolates in your local fungal populations, because these can cause significant crop loss on all current chickpea cultivars.
The research shows that:
- The chickpea Ascochyta Blight fungus population is evolving to select increased frequencies of highly aggressive isolates.
- More aggressive isolates have contributed to increased susceptibility of all cultivars.
- When fungicides are used according to label rates and schedules recommended, they effectively protect the crop.
- We can slow down the rate of pathogen evolution by adhering to appropriate management practices such as fungicides and sensible rotations.
Background
Ascochyta Blight (AB), caused by the fungus Ascochyta rabiei (syn Phoma rabiei), is a major endemic disease of the Australian chickpea industry, resulting in significant crop loss and management costs. The GRDC project “Improving grower surveillance, management, epidemiology knowledge and tools to manage crop disease; national chickpea pathology program” (UM00052) has provided data on the variation and distribution of isolates and their aggressiveness across growing regions.
This information is used to improve disease management strategies, including using the worst (most aggressive) isolates for use in screening in the PBA chickpea program’s resistance breeding. However, higher frequencies of aggressive isolates have resulted in increased disease severity on broadly grown cultivars, including on PBA Seamer. The resultant resistance erosion in PBA HatTrick is due to an increase in the number of highly aggressive isolates in the region. This is likely caused through environmental factors that impose selective pressure on the fungal population, including cultivar choice and non-optimal fungicide usage as well as inoculum build-up from minimal to no rotation.
We have a situation - evidence of a growing number of highly aggressive isolates
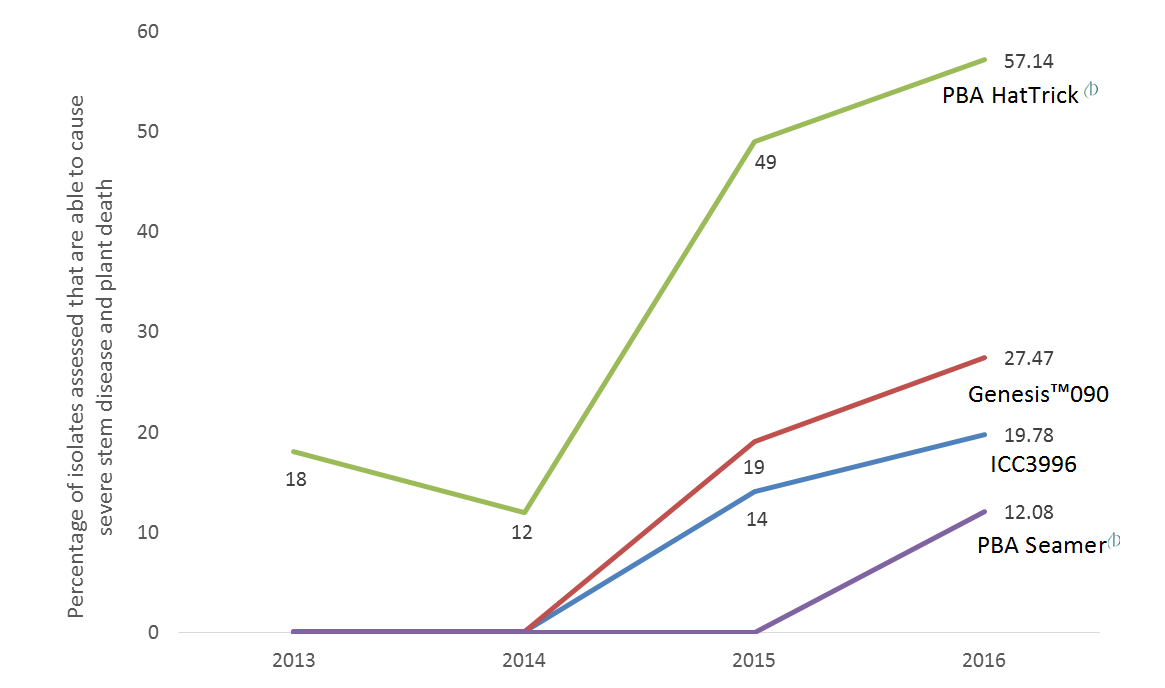
Over the past four years, within GRDC project UM00052, a sub-set of isolates were collected annually from all major growing regions across Australia where AB epidemics have occurred. The ability of these isolates to cause severe disease on a set of resistant lines was then measured in plant growth chambers. From this, it is clear that since 2015, a significant increase in numbers of isolates able to cause severe damage on our formally “resistant” cultivars has occurred. In particular, the proportion of isolates that are highly aggressive on PBA HatTrick has risen from 18% in 2013 to 57% in 2016. Meanwhile, we also see more isolates able to cause severe disease on Genesis™090, a major cultivar grown in southern Australia, and even on ICC3996, a commonly used resistance breeding source. Of most concern for northern growers is the 12% of isolates able to cause severe disease on PBA Seamer (Figure 1).
When PBA Seamer was released, it was rated “resistant” to AB, (but by no means immune); in 2017 AB caused severe damage on PBA Seamer (back to back in Kyabra residue and no foliar fungicides), and had it not stopped raining at the end of July, the crop would have been lost. It is highly likely more isolates capable of causing severe damage on PBA Seamer will evolve.
The increase in aggressiveness seen in this long-term study has likely occurred through “pathogen adaptation” - in other words, the fungus has evolved to make more highly aggressive isolates due to “selective pressures” being applied through environmental factors and farming practices. Whilst we often cannot control external environmental factors, we can reduce selection towards evolution of increased aggressiveness by adhering to recommendations of crop rotation and fungicide use.
Figure 1. The percentage of isolates screened in controlled environment bioassays that were able to cause severe disease on the four host chickpea lines (90-110 isolates screened per year).
Evidence of fungicide efficacy
To demonstrate that fungicides, when used appropriately are effective in protecting the crop from the most aggressive isolates of AB, trials were conducted at Turretfield, SA in 2015 and 2016. Seeds were dressed with P-Pickel T® at 200 mL/100kg, fertilised with MAP +Zn (2%) at 90 kg/ha at a sowing density of 50 plants/ m2 (desi) and 35 plants/ m2 (kabuli). Crops were sprayed fortnightly with chlorothalonil or not sprayed. Limitations exist as to the maximum number of chlorothalonil sprays and/or total volume of product that can be used in a crop. Read and adhere to label directions.
The inoculum was Ascochyta infested chickpea stubble collected from SA chickpea trials in 2015 and from SA trials and commercial crops in 2016. The study clearly showed that fungicide treatment is absolutely necessary for many of the older varieties; in contrast chlorothalonil provided no or little benefit on several of the newer CICA lines (Table 1). This highlights the need to adopt the correct recommended chemical and spray regime for the specific cultivar that is grown.
Meanwhile, as the fungal population increases in aggressiveness, the disease rating of each cultivar must be reassessed on an annual basis to maintain accuracy for informing disease management strategies. In 2015, several cultivars showed good resistance to AB (i.e. Genesis™090 and Genesis™079). In 2016, high disease severity was observed across all lines, with significant yield losses (i.e. Genesis™090 had ~30% disease severity and 30% yield loss, although it is still considered the best of the commercial cultivars in the southern region). All commercial lines used in southern growing regions are now considered S or MS to AB.
Table 1. The impact on yield from regular chlorothalonil treatment on a range of chickpea cultivars and advanced breeding lines, and recent changes to cultivar disease ratings (southern region).
Line | Yield with fungicide (t/ha) | Yield no fungicide (t/ha) | Yield loss (%) | Plant Disease Index (un- sprayed plots) | AB foliar disease rating before 2016 | AB foliar disease rating 2017 |
|---|---|---|---|---|---|---|
CICA1454 | 2.43 | 2.50 | -3 | 22 | - | - |
CICA1551 | 2.31 | 1.98 | 14 | 28 | - | - |
CICA1541 | 2.69 | 1.81 | 33 | 35 | - | - |
Genesis™090 | 2.24 | 1.61 | 28 | 37 | R | MS |
PBA Slasher | 3.10 | 1.60 | 48 | 51 | R | MS |
Neelam | 2.86 | 1.46 | 49 | 57 | R | MS |
Almaz | 1.94 | 1.01 | 48 | 63 | MS | MS |
Genesis™079 | 2.56 | 0.87 | 66 | 57 | R | S |
PBA Striker | 2.74 | 0.28 | 90 | 81 | MR | S |
Sonali | 2.69 | 0.14 | 95 | 79 | S | S |
PBA Monarch | 2.27 | 0.11 | 95 | 87 | MR | S |
Varietal ratings to AB have also changed in northern growing regions under a separate rating system, demonstrating that evolution of the pathogen is occurring at a national level (Table 2).
Table 2. Changes in Ascochyta ratings (northern region) for selected chickpea varieties from time of release to 2017.
Variety | Desi/Kabuli | Release year | Release AB rating | Revised AB rating (Feb 2017) |
|---|---|---|---|---|
Kyabra | Desi | 2005 | VS | VS (no change) |
Flipper | Desi | 2005 | MR | S |
PBA HatTrick | Desi | 2009 | MR | MS |
PBA Boundary | Desi | 2011 | MR | MS |
PBA Seamer | Desi | 2016 | R | MR |
Almaz | Kabuli | 2005 | MS | MR/MS |
Genesis™090 | Kabuli | 2005 | R | MR |
Genesis™425 | Kabuli | 2007 | R | MR |
Genesis Kalkee | Kabuli | 2011 | MS | MS |
PBA Monarch | Kabuli | 2013 | MS | MS (no change) |
Hope prevails - breeding for AB resistance
To identify material that is the most resistant to the pathogen, entries in the PBA Chickpea Stage 3 trials that show desirable disease and agronomic traits and may progress to be released varieties, as well as existing cultivars, are screened annually with the WORST isolates identified in the previous growing season. Disease severity is measured as the mean % main stem breakage under shade house trials performed at SARDI. The breeding program has developed material with better resistance than PBA Seamer to isolates from northern NSW/Qld (ie Yallaroi and Narromine) (Table 3).
Table 3. The disease reaction of cultivars and advanced breeding material to the worst (most aggressive) isolates identified annually. Where dark grey = >50% of stems broken, light grey = ≤ profit 49.99 % and >10% stems broken and white = ≤10% stems broken.
Cultivar/line and isolate used | 2017 | 2017 | 2016 | 2016 | 2015 | 2015 | 2014 | 2014 |
|---|---|---|---|---|---|---|---|---|
JIMBOUR | 100 | 100 | NOT TESTED | NOT TESTED | 100 | 100 | 92 | 100 |
KYABRA | 100 | 100 | NOT TESTED | NOT TESTED | 100 | 100 | 100 | 100 |
PBA BOUNDARY | 100 | 100 | 75 | 66.67 | 35 | 75 | 8 | 8 |
PBA HATTRICK | 92 | 92 | 83.33 | 68.33 | 0 | 25 | 0 | 0 |
PBA MONARCH | 28 | 20 | 58.33 | 58.33 | 3.33 | 41.67 | 0 | 0 |
PBA PISTOL | 100 | 100 | NOT TESTED | NOT TESTED | 91.67 | 83.33 | 67 | 58 |
PBA SEAMER | 87 | 75 | 35 | 33.33 | 0 | 41.67 | 0 | 0 |
CICA1303 | 100 | 100 | 91.67 | 100 | 6.67 | 91.67 | 8 | 0 |
CICA1521 | 75 | 75 | 26.67 | 43.33 | 0 | 8.33 | NOT TESTED | NOT TESTED |
CICA1720 | 51.67 | 20 | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED |
CICA1721 | 30 | 41.67 | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED |
D10016> | 3.33 | 6.67 | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED | NOT TESTED |
GENESIS™090 | 35 | 12 | 6.67 | 47 | 0 | 8.33 | 0 | 0 |
GENESIS KALKEE | 60 | 60 | 35 | 31.67 | 50 | 20 | 0 | 0 |
CICA1156 | 50 | 6.67 | 6.67 | 33.33 | 0 | 0 | 0 | 0 |
CICA1352 | 28.83 | 20 | 48.33 | 38.33 | 0 | 8.33 | 0 | 0 |
CICA1454 | 28.33 | 13.33 | 3.33 | 20 | 0 | 0 | 0 | 0 |
CICA1751 | 10 | 0 | 3.33 | 11.67 | 0 | 0 | 0 | 0 |
In summary
To prolong cultivar lifespans (i.e. PBA Seamer) and maintain profitability, the following best management practices must be followed by all growers:
- Treat all seed with a thiram based fungicide that is registered for use in chickpeas to control seed transmission of Ascochyta Blight.
- Apply strategic fungicide sprays to MS cultivars that offer 2-3 weeks of protection each. Apply these ahead of rain events starting before the first post emergence rain event.
- Spray susceptible cultivars before every rain event, unless the last application was less than 14 days ago.
- Apply fungicide at pod set before rainfall events, as the pods of all cultivars are more susceptible than vegetative plant parts and pod infection can lead to seed staining and seed abortion.
- Observe a minimum of a three-year rotation between chickpeas in the same paddock and avoid planting adjacent to the previous year’s chickpea. This is because the fungus can survive on stubble and volunteers.
One last point to consider
Do not be complacent; if you follow best disease management practice, you will reduce the AB risk to yourself and your neighbours. The fungus is already present in your region and an epidemic will happen with optimal conditions, selective mutation and sufficient inoculum. Judicious integrated disease management will avoid crop failure and ensure a profitable outcome.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the investment of the GRDC, the authors would like to thank them for their continued support.
Contact details
Rebecca Ford
Griffith University
170 Kessels Rd, Nathan, Queensland 4210
Ph: 07 3735 5066
rebecca.ford@griffith.edu.au
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
®Registered trademark
TM Trademark
Was this page helpful?
YOUR FEEDBACK