Helicoverpa armigera resistance management in pulses, and recent research findings on Rutherglen bug
Author: Melina Miles, Adam Quade, Trevor Volp, DAF Queensland | Date: 23 Jul 2018
Take home messages
- The H. armigera resistance management strategy is designed to prolong the useful life of the newer chemistry currently available to pulse growers. Familiarise yourself with the strategy and the full range of options available for Helicoverpa control in chickpeas, mungbeans and soybeans. Consider what products you will use if a second spray is required in these crops.
- Rutherglen bug adults are present in canola crops much earlier than was previously thought. Females are depositing eggs in the soil and leaf litter from early spring through to harvest. At this point, there is no obvious option for preventing the build-up of large populations of nymphs in canola stubble, but recent work is helping to understand how these populations develop.
The Helicoverpa armigera resistance management strategy (RMS)
This material has been extracted from the Science behind the strategy document.
General rationale for the design of the strategy
Chickpeas and mungbeans are currently, and for the foreseeable future, the most valuable grains crops influenced by the RMS. Therefore, the resistance management strategy (RMS) is primarily focused on insecticide Modes of Action (MoA) rotation in these systems and is built around product windows for Altacor® and Steward® because:
1. Altacor® (chlorantraniliprole) is at risk from over-reliance in pulses, but resistance frequencies are currently low.
2. Steward® (indoxacarb) is at risk due to genetic predisposition (high level genetic dominance and metabolic mechanism) and pre-existing levels of resistance in NSW and QLD (with elevated levels in CQ during 2016-17). In addition, the use of indoxacarb in pulses may increase as generic products come on to the market.
There are two regions within the RMS, each with their own resistance management strategy designed to make the most effective products available when they are of greatest benefit, whilst minimising the risk of overuse:
1. Northern Grains Region: Belyando, Callide, Central Highlands & Dawson (Table 1)
2. Central Grains Region: Balonne, Bourke, Burnett, Darling Downs, Gwydir, Lachlan, Macintyre, Macquarie & Namoi (Table 2)
- The RMS provides windows-based recommendations common to these regions because H. armigera moths are highly mobile and have the capacity to move between these regions.
- No RMS is currently proposed for the Southern and Western grain regions (Victoria, South Australia and Western Australia) for winter crops. Biological indicators suggest that that the risk of H. armigera occurring in winter crops, at densities where control failures may occur, is presently considered low. Helicoverpa control in summer crops in these regions should use the Central Grains region RMS.
Use of broad-spectrum insecticides
The early use of synthetic pyrethroids (SPs) in winter pulses (August – early September) is adopted where the assumption is made that early infestations of Helicoverpa will be predominantly H. punctigera which are susceptible to SPs. Similarly, the use of carbamates to delay the application of Group 28 or Group 6 products, carries risks. If adopting this strategy, be aware of the following risks:
- Recent monitoring with pheromone traps has shown H. armigera to be present in all parts of the Northern Grains region from early August (www.thebeatsheet.com.au).
- Reduced efficacy of SPs and carbamates against H. armigera can be masked when treating very low population densities (< 3/sqm).
- If H. armigera are present, even at low levels in a population treated with SPs or carbamates, the treatment will select for further resistance. Whilst initial applications may be effective, later treatments may be significantly less effective.
Table 1. Grains resistance management strategy for Helicoverpa armigera across Australia. Best practice product windows and use restrictions to manage insecticide resistance in H.armigera.
Northern Region: Belyando, Central Highlands, Dawson and Callide.
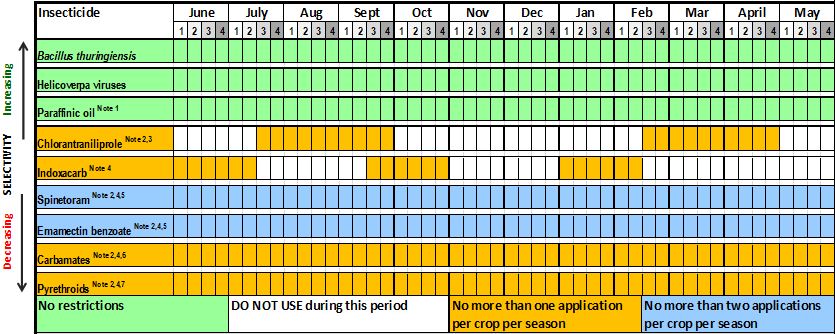
Notes:
- Some nC27 paraffinic spray oils can be used to suppress Helicoverpa populations and are best used as part of an IPM program.
- Observe withholding periods (WHP). Products in this group have WHP 14 days or longer.
- Maximum one spray of chlorantraniliprole alone or in mixtures per crop per season.
- Refer to label for warning of insecticide risk to bee populations.
- Maximum two consecutive sprays alone or in mixtures per crop per season.
- MODERATE RESISTANCE IS PRESENT IN H.ARMIGERA POPULATIONS – FIELD FAILURES LIKELY.
- HIGH RESISTANCE IS PRESENT IN H.ARMIGERA POPULATIONS – FIELD FAILURES EXPECTED!
Table 2. Grains resistance management strategy for Helicoverpa armigera across Australia. Best practice product windows and use restrictions to manage insecticide resistance in H.armigera.
Southern QLD, Central & Northern NSW Regions: Balonne, Bourke, Burnett, Darling Downs, Gwydir, Lachlan, Macintyre, Macquarie & Namoi.
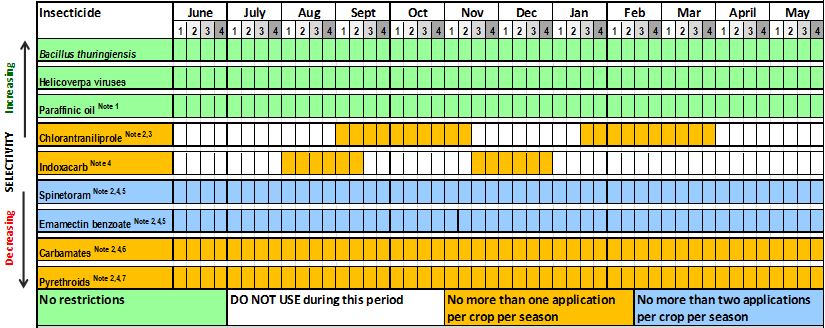
Notes:
- Some nC27 paraffinic spray oils can be used to suppress Helicoverpa populations and are best used as part of an IPM program.
- Observe withholding periods (WHP). Products in this group have WHP 14 days or longer.
- Maximum one spray of chlorantraniliprole alone or in mixtures per crop per season.
- Refer to label for warning of insecticide risk to bee populations.
- Maximum two consecutive sprays alone or in mixtures per crop per season.
- MODERATE RESISTANCE IS PRESENT IN H.ARMIGERA POPULATIONS – FIELD FAILURES LIKELY.
- HIGH RESISTANCE IS PRESENT IN H.ARMIGERA POPULATIONS – FIELD FAILURES EXPECTED!
Table 3. Explanatory notes for product windows in all regions.
Insecticide | Number of insecticide windows | Duration of insecticide windows | Maximum number of applications/crop/season |
|---|---|---|---|
Chlorantraniliprole (Altacor®) | 2 | 10 weeks | 1 |
| |||
Indoxacarb (e.g. Steward®) | Northern – 3 Central – 2 | 6 weeks | 1 |
| |||
Bacillus thuringiensis | 1 | Season long | No restrictions No restrictions 2 |
Helicoverpa viruses | |||
Spinetoram (e.g. Success Neo®)* | |||
| |||
Emamectin benzoate (e.g. Affirm®)* | 1 | Season long | 2 |
| |||
Carbamates | 1 | Season long | 1 |
Synthetic pyrethroids | |||
| |||
*Resistance monitoring for selective products is a key component of the RMS and changes in resistance frequencies may result in the introduction of product windows for those insecticides not currently windowed.
The number of uses in the RMS is more restrictive than stated on the Altacor® label, why?
To avoid repeated use of either Steward® or Altacor® within the use window, the number of allowable applications is 1 per crop. Whilst this is currently inconsistent with the Altacor label (2 applications per crop), we expect that there will be changes to the label to ensure consistency in these recommendations.
Does the RMS impact on recommendations for insecticide use in cotton and other crops?
The RMS is not intended to compromise the ability of the cotton industry to use any products registered for Helicoverpa in Bollgard® cotton. This is because selection for insecticide resistance is considered low due to the high likelihood that survivors of conventional sprays used in Bollgard cotton would be killed by Bt toxins expressed in plants. For further information go to CottonInfo's pest management guide.
Similarly, the RMS does not attempt to align the use of the Group 28s in mungbeans and chickpeas with use in other grain crops or horticulture. To do so would add a level of complexity that would make the RMS impractical.
Shouldn’t other modes of action (MoA) be windowed to prevent the potential development of resistance to these products?
There is little evidence to suggest that other products should be windowed now to slow the development of future resistance. Both Affirm® (emamectin benzoate) and Success Neo® (spinetoram) show no sign of reduced susceptibility in testing (L. Bird, CRDC data). This result is consistent with the relatively limited use these products in the grains industry to date. If a shift in susceptibility is detected in future testing, it is the intention that the product/s will be windowed to limit selection pressure.
The SPs and carbamates are not windowed because there is already well established, relatively stable moderate-high levels of resistance to these MoAs, and limiting their use will not change this situation.
By restricting the use of just the ‘at risk’ products, keeping the RMS as simple as possible, and allowing maximum choice of registered products we anticipate that the grains industry will be more inclined to use the RMS.
What is the relative efficacy of the ‘softer’ options for Helicoverpa control in mungbeans and chickpeas?
In 2017, QDAF entomology undertook a number of trials to compare the knockdown/contact efficacy, and residual efficacy (persistence in the crop) of Altacor®, Steward®, Affirm® and Success Neo®. The purpose of these trials was to provide agronomists and growers with information on how well each of the products worked, and to provide confidence to use another option, rather than relying solely on the Group 28 products.
The results show that these products are equally effective on 3rd, 4th or 5th instar larvae that receive a lethal dose of the product – as would be achieved with good spray coverage (Figure 1a). However, there is considerable benefit in products persisting in the crop to control larvae that may hatch after the spray, or emerge from flowers, buds or pods where they may have been protected from an earlier applciation. The long residual efficacy Altacor ® has been a major factor in its popularity. The data in Figure 1b shows the relative efficacy of these products from 0 – 20 days after treatment in the field (at 5 day intervals).
For more information on the relative performance of these products in terms of feeding potential and recognising larvae affected by the different insecticides, see recent articles on the Beatsheet blog (www.thebeatsheet.com.au).
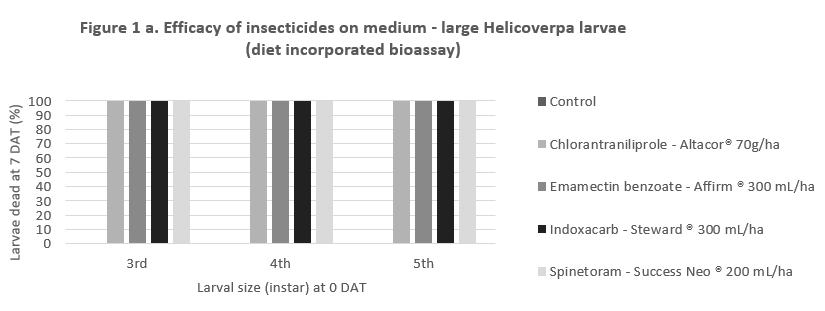
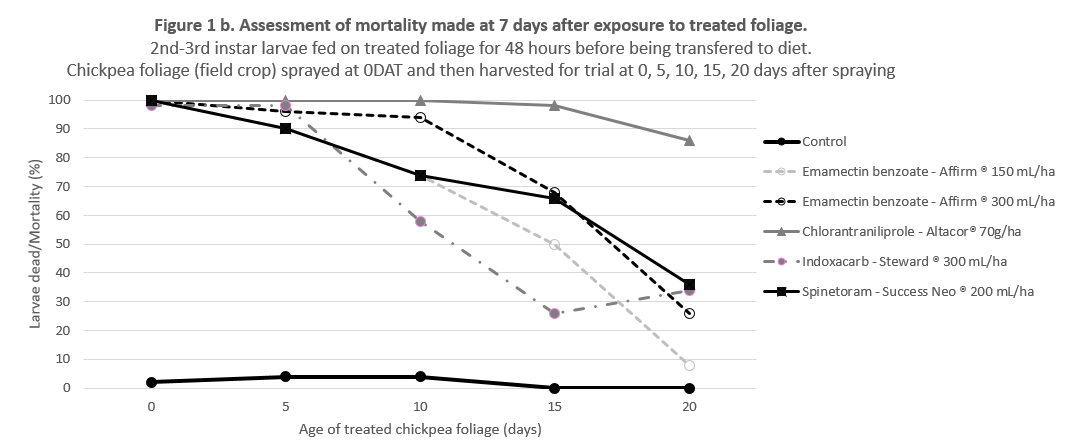
Figure 1. Relative efficacy (a) direct contact and (b) residual, of softer options for Helicoverpa control in chickpea and mungbean crops.
Rutherglen bug build up in canola – population dynamics during the 2017 season
In recent seasons, higher densities of Rutherglen bug (RGB) have been experienced. One of the challenges of this higher RGB pressure has been the movement of large numbers of nymphs from canola stubble into neighbouring summer crops. Through sheer weight of numbers, RGB nymphs can kill sorghum, cotton, soybean, corn and sunflower plants in the rows closest to canola. The movement of nymphs can occur over a period of weeks, and even regular spraying of the affected crops may not prevent significant crop loss.
Understanding how these enormous populations of nymphs develop is key to working out how they might be prevented, or managed, so that they don’t affect neighbouring summer crops. Rather than focusing on controlling the nymphs, we were interested in whether there may be an opportunity to control the adults before they reproduce. During the spring of 2017, QDAF entomology monitored a number of canola crops, from the Darling Downs to the Liverpool Plains. We assessed the density of adults in the canola, dissected females to determine if they were reproductive (laying eggs), and assessed the crops for nymphs. We also attempted to determine the timing of egg laying by assessing the density of eggs – however, we were unable to do this effectively. Other than determining that eggs are deposited in the soil and on the leaf litter on the soil surface (not in the crop canopy), we could not reliably assess egg density.
Whilst this is only one season of data, it is presented here to highlight the following key findings.
RGB adults were present in the canola crops much earlier than we expected (Figure 2). Even at the most southerly site (Curlewis, NSW), RGB adults were present in canola from early August. At most sites, numbers increased through September and October.
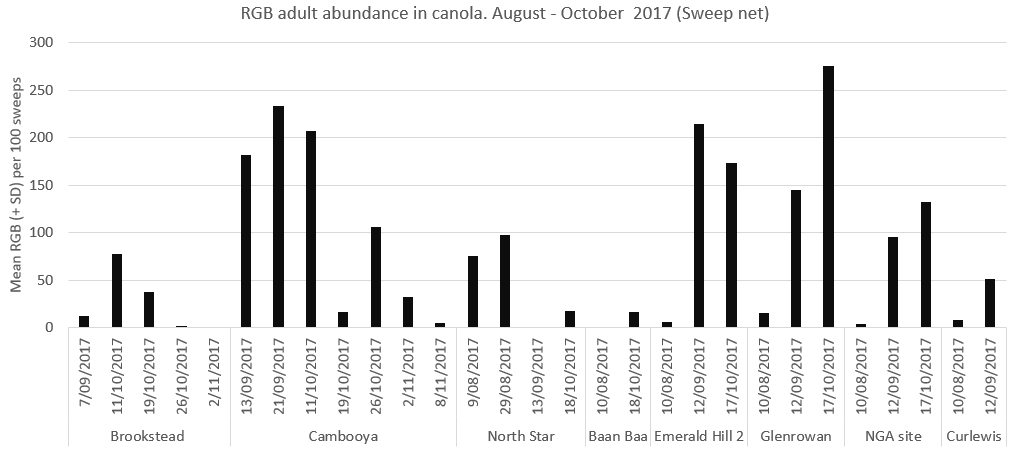
Figure 2. RGB adults were present in canola from late winter (August – September).
(SD = standard deviation)
Female RGB were reproductive (had mature eggs in their ovaries ready to lay) from September onwards, and the percentage of the population that was reproductive increased from September through November (Figure 3).
Although the majority of female RGB were reproductive, and laying eggs, we did not see nymphs start to emerge until much later than expected based on the day degrees accumulated during this period (Figure 4).
It is possible that the development of eggs is slowed by the relatively cool temperatures experienced on the soil surface under the leaf litter and crop canopy. When the crop is harvested or windrowed, the temperature of the soil quickly rises, potentially resulting in synchronous hatching of eggs that have been laid over a period of 2-3 months.
More data is needed over additional sites and seasons to confirm our theory, and closer monitoring of soil temperature and RGB egg development is also needed to understand exactly what is happening.
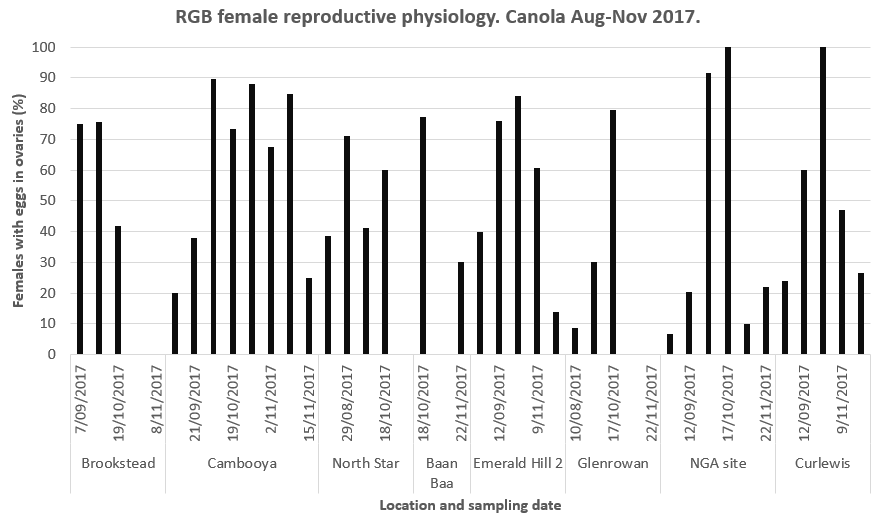
Figure 3. A high proportion of the female RGB sampled were laying eggs from September through November.
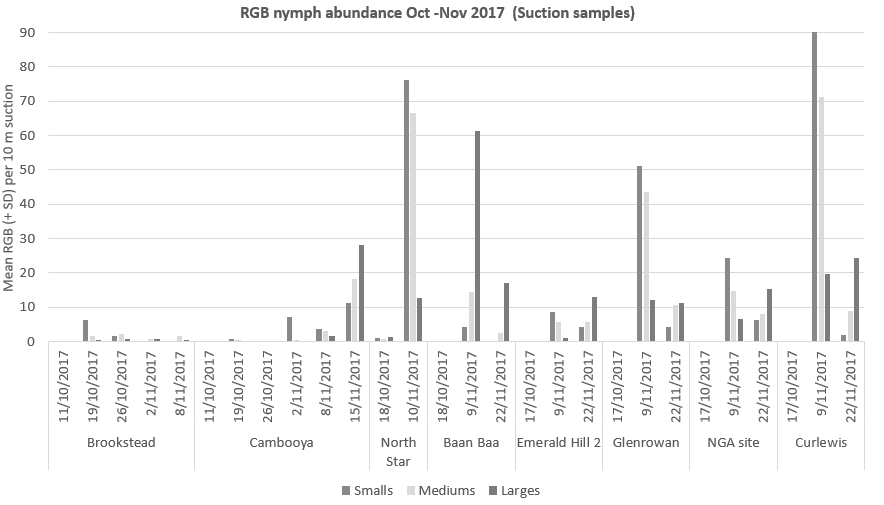
Figure 4. Nymphs did not start to emerge until much later than expected based on the day degrees accumulated during this period, despite the majority of female RGB being reproductive
and laying eggs.
The take home message from this RGB work is that there doesn’t seem to be an easy fix to prevent the build-up of RGB nymphs in canola stubble. The long period of egg laying by the females, and the potential challenges with controlling nymphs on the ground under the crop canopy, means that there is no obvious opportunity to prevent the population build up.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
Information on the H. armigera RMS is extracted from material prepared by NIRM to support the implementation of the RMS. The authors acknowledge the contribution of NIRM members to the development of this material.
We are grateful to the growers who allow us access to their farms and crops, and to the agronomists who assist us in locating potential field sites. We also thank the many growers and agronomists who share with us their experiences and insights into the issues they face and the practicalities of the management options we propose.
Reference
NIRM (2018) Science behind the Resistance Management Strategy for Helicoverpa armigera in Australian grains.
Contact details
Melina Miles
Queensland Department of Agriculture and Fisheries
203 Tor St, Toowoomba. QLD 4350
Ph: 0407 113 306
Email: melina.miles@daf.qld.gov.au
® Registered trademark
Was this page helpful?
YOUR FEEDBACK
