Current management advice for Russian wheat aphid and Green peach aphid
Author: Elia Pirtle, James Maino, Jessica Lye, Paul Umina and Lisa Kirkland (cesar Pty Ltd), Thomas Heddle and Maarten van Helden (South Australian Research and Development Institute) | Date: 26 Feb 2019
Take home messages
Russian wheat aphid
- Surveys have shown that Russian wheat aphid (RWA) is distributed throughout Australian cereal growing regions. While population numbers have declined over summer, preliminary data suggests their overall range may remain the same, due to green bridge reservoirs in towns and irrigated regions. It is advised to monitor for the aphid itself on green bridge hosts, particularly in favourable hosts like barley grass and brome grass, as classic RWA symptoms have been rarely observed on weed species over spring and summer. Volunteer cereals and weedy grasses (particularly barley and brome grasses) found within next season’s cereal paddocks should be controlled at least 2-3 weeks prior to sowing.
● To ensure seed treatments remain a long-term viable control option for RWA, growers are urged to use neonicotinoid seed treatments judiciously and according to the regional risk, and using the Find, Identify, Threshold, Enact (FITE) approach.
○ The overseas threshold >20% of all plants infested up to GS30 and >10% of tillers infested from late stem elongation (GS30 or later) is recommended.
● RWA is controlled by many beneficials, including parasitoid wasps which were active in many of the aphid populations detected in spring. Monitor aphid populations for signs of parasitism and consider control options that will have minimal impact on beneficial populations.
Green peach aphid
● Insecticide resistance continues to increase in Australian green peach aphid (GPA) populations, including resistance to neonicotinoids.
● The implementation of recently published resistance management strategies is vital and should be adhered to wherever possible.
● It is important to use a diversity of management practices, and rotate insecticide groups, to prolong the viability of important aphicides such as sulfoxaflor.
Background
Two key aphid pests of cereals
Green peach aphid (Myzus persicae)
GPA originates from China but is now globally distributed and present in Australia. It is a polyphagous pest that can eat a variety of different plant types including canola. It will also feed on grasses, as well as cucurbits, solanaceae and other Brassica species. This means it is also a pest of other industries, such as horticulture, which has implications for resistance management (as insectides are also used in other industries and resistant aphids can spread between these industries and cereals). It is a prolific virus vector and can transmit more than 100 different plant viruses, including Turnip Yellows Virus (TuYV). Damage can also occur from feeding as populations build up, and accumulation of the honey dew the aphids produce can lead to sooty mould build-up.
Like most insects, GPA is highly influenced by temperature and rainfall, which can affect population densities and time of aphid flights. Hot, arid conditions limit aphid survival and GPA thrives in milder conditions over autumn and spring, so peak activity occurs over these times.
Within Australia, GPA mostly reproduces asexually, meaning it can increase its numbers very quickly, making the development of resistance a major issue. GPA has developed resistance to 74 insecticides with different modes of action, as classified by the Insecticide Resistance Action Committee (IRAC).
Russian wheat aphid (Diuraphis noxia)
RWA is one of the world’s most economically important and invasive pests of wheat, barley and other cereal grains. Since first being discovered in South Australia (SA) in 2016, RWA has been found widespread in cereal growing regions of SA, Victoria, New South Wales (NSW) and Tasmania. Their small size, green colour, elongated shape, very short antennae and apparent lack of siphuncles readily distinguish RWA from other pest aphids found in Australian cereal crops. Unlike other aphids, which cause damage through feeding on plant nutrients, RWA injects salivary toxins during feeding that cause rapid, systemic phytotoxic effects on plants, resulting in acute and observable plant symptoms, as well as potentially significant yield losses.
The first detection of RWA in Australia occurred on cereal crops in May 2016. Within one month, a combined industry-government biosecurity committee determined that an eradication attempt for RWA was unlikely to be successful. RWA is now a management concern for grain growers in regions where it has been found.
In a recent study by Avila et al. (2019), the potential spread and establishment of RWA in Australasia were assessed using a re-parameterised CLIMEX model that considered currently known distribution records of the aphid and the presence of irrigated crops. According to the model results, RWA has the potential to establish in all key grain growing regions in Australia. However, since RWA has not been previously detected in Australia, it is not yet known what effect local agro-climatic conditions will have on the ability of RWA to establish and feed on hosts, which include wheat, barley and a wide range of cultivated and wild grasses.
Ongoing research
Aphid activity over the summer and implications for regional risk
RWA and the summer green bridge (GRDC 9176535)
There are many factors that will influence aphid survival during times when favoured crop hosts are not available for nourishment, including local climate, land use (e.g. vegetation on roadsides, irrigated public spaces), availability of alternate hosts, abundance of volunteer cereals, and predation by beneficials. Understanding how these environmental factors contribute to aphid population survival over the summer aids in proactive aphid management to protect emerging crops the following autumn.
A new GRDC investment (9176535), ‘Russian wheat aphid risk assessment and regional thresholds’ has been launched to investigate regional risk and management tactics for RWA, as well as to better understand the role that green bridges play in supporting RWA populations between cereal cropping periods. The South Australian Research & Development Institute (SARDI) and cesar are currently conducting surveillance in SA, Victoria, and NSW over spring and summer from October 2018 to February 2020. It is generating data about types of vegetation the aphid is surviving on between cropping, and what environmental conditions support its survival over this period, as well as collecting valuable information about beneficial species predation of RWA. Once enough green bridge data is collected, use of modelling algorithms will allow us to predict aphid population growth over this critical period (the modelling is not a focus of this update paper).
Where has RWA been found?
Our most current data indicates that RWA is present in a large, and still expanding, area covering all cereal growing regions of SA, Victoria, Tasmania and most of NSW. Spring sampling shows that the aphid is widespread across these regions in at least low numbers, however it is not known how typical this spring distribution is as we have only sampled for one season. In late 2018, the aphid was detected at Coonabarabran and the Liverpool Plains (NSW), which is a northerly extension of range for this aphid.
A distribution map that is still commonly used to understand and demonstrate RWA distribution in Australia is derived from AusPestCheck (Plant Health Australia), which collected monitoring data from state governments when RWA was under active surveillance by biosecurity authorities. Through the current project, an RWA portal has been produced which includes an up-to-date map that sources data from 2018 green bridge surveillance and adviser reports to PestFacts services. This map updates in real time displaying RWA presence and absence points. Users can toggle the timeframe between 2016 and 2019. This map can be found on the RWA Portal (Figure 1, also see Resources section).
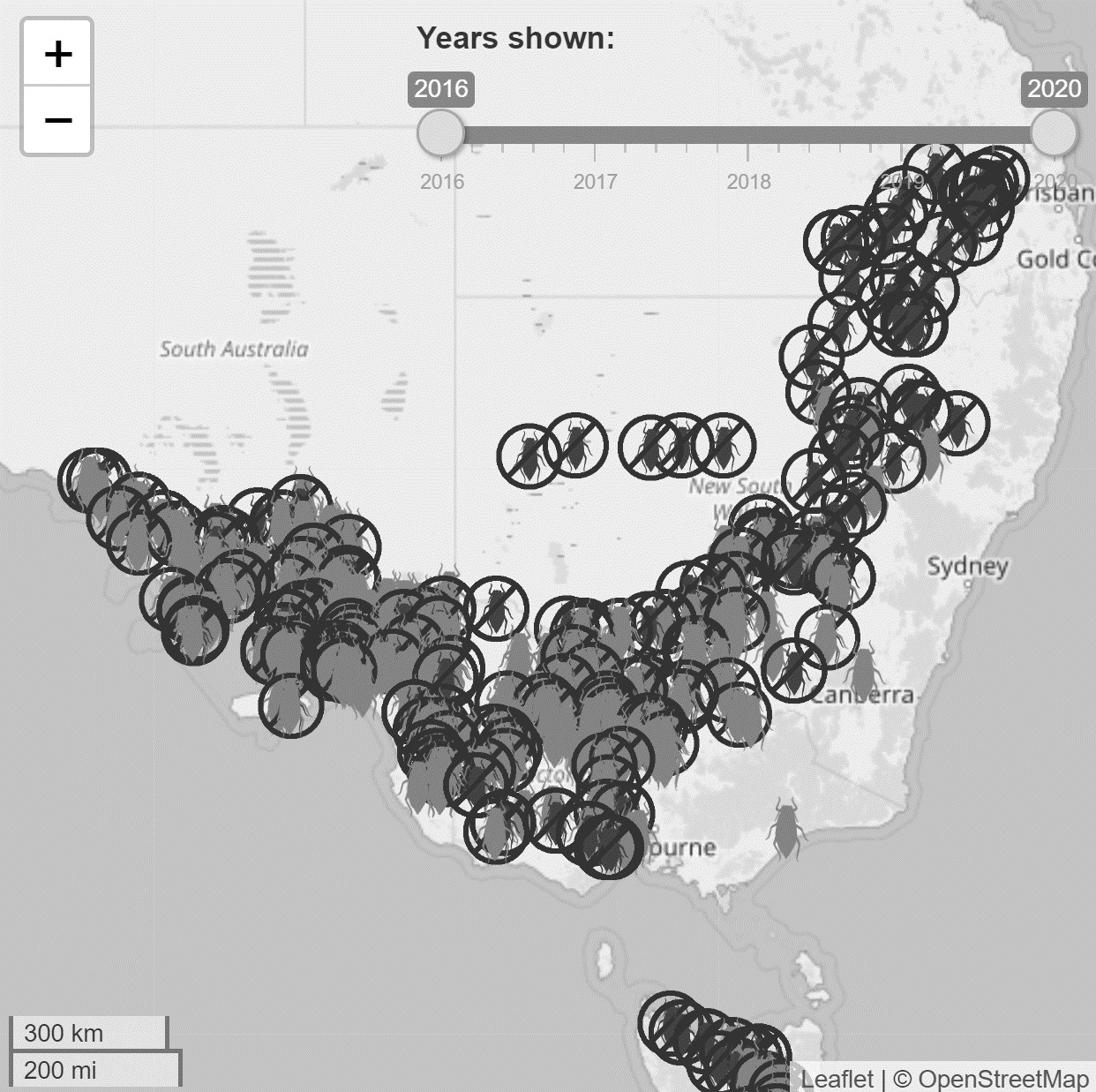
Figure 1. RWA interactive map. Detections span 2016-2018 with data sourced from 2018 green bridge surveillance and adviser reports to PestFacts. The darker crossed out aphid icons denote RWA absences; lighter aphid icons denote RWA presences. (Map developer: Dr James Maino, cesar).
What we know about the environmental conditions under which RWA will thrive?
Despite few RWA issues reported to PestFacts services during the 2018 cereal growing season, spring sampling detected RWA in all cereal growing regions where RWA has been reported previously. The presence of RWA in an area does not necessarily mean it will impact crop yield. RWA needs to infest cereals in early autumn in order to develop into damaging population levels in spring during booting and flowering.
While data about conditions that support RWA survival is still being accrued and only limited advice can be given, here is what can be said:
- Hot and dry summer conditions reduce over-summering populations of the aphid, with RWA likely to persist over the summer where there is available moisture and green host plant material (from rainfall or irrigation).
- Higher than average temperatures are unfavourable for RWA survival.
- Localised summer rainfall events, resulting in germination of weeds such as barley grass, can provide refuges for the aphid.
- Field observations and experiments over the past three seasons indicate that RWA abundance and development on crops during the growing season are much higher in low rainfall zones (<400mm per year) and on drought stressed crops. However, these areas may become more unsuitable during the summer, as preferred hosts dry up, than higher rainfall areas where preferred hosts can persist.
- This year’s field trial observations support international research findings that indicate mature crops (GS40 or higher) are less attractive and are less likely to be invaded by RWA in spring.
This work is ongoing – RWA is still a very new pest to Australia and we are continuing to learn about its biology as the current investment progresses. More pertinent information about environmental influences is likely to be gained at crop establishment, particularly in area-wide aphid abundance and flight timing. Significant early infestation of a crop will only occur through a combination of abundant green bridge and good flight conditions that would aid RWA migration to cereal paddocks during the seedling stage in early autumn. Good flight conditions for aphids are calm, warm days over 20°C. These conditions were not met over the 2018 season in southern Australia.
What is the influence of region, season and local conditions on RWA populations?
SARDI and cesar have been sampling RWA over spring, with summer time follow up surveys currently in progress, to determine what conditions support survival of the aphid leading into autumn sowing. Data analysis is ongoing, but some early conclusions can be drawn regarding RWA abundance across region and season.
During spring, RWA populations were found to be widespread in Victoria, NSW and SA, consistently appearing in randomly selected roadside stops, regardless of proximity of cereal crops or sources of water. The limiting factor for their presence seemed to be the presence of preferred host weed species. At most sites, populations of RWA were found residing in weeds outside of crops, however these populations were generally smaller than those found within crops. Within crops, large populations causing visible symptoms were most commonly observed in young tillers, particularly on paddock edges.
Populations in southern Victoria and Tasmania were comparatively sparse. In Tasmania during the spring, aphids were largely restricted to crops despite the abundance of green host weeds that were observed to be preferred in Victoria and NSW (such as barley grass). It is unclear if the lack of positive detections of RWA from the north-western regions of Tasmania and the southern regions of Victoria are due to populations being too diluted among the plentiful green vegetation or are due to unsuitability or incomplete dispersal.
While summer abundance data is still preliminary as surveys are currently in progress, early insights from the Victorian sites suggest that RWA populations have declined dramatically over the summer, however they are still present throughout Victoria (see Figure 2). The few active populations that were detected were again strongly dependent on the presence of preferred hosts such as barley grass, which had become far less common. Summertime RWA populations were also more strongly associated with the presence of persistent sources of water that could maintain green host plants than they were in the spring. Three of the five active populations detected during the summer in Victoria were found within city limits, in areas that would receive relatively consistent summer watering, including weedy lawns and ovals. Over-summering RWA populations were also detected on regrowth within cereal paddocks in the cooler, southern regions of Victoria (interestingly, in areas where they were not detected during spring).
Data for SA is still being compiled, together with surveys in NSW.

Figure 2. RWA population distribution and abundances across Victoria in spring (left) compared to summer (right). Presences are represented by light aphid icons, and absences by dark crossed out aphid icons.
Tasmanian summer data collected so far is showing a different story, with RWA populations being slightly larger than they were in the spring, or at least more easily detected due to fewer green crops and grasses available to sample. More populations were detected on weeds than in spring, which again could be due to the dilution of populations on abundant green weed material in the spring reducing detection likelihood, compared to fewer green weed options in the summer.
Which weeds and summer pasture species supported RWA over summer?
During the 2018 spring sampling, RWA was found on a variety of non-crop grasses, with barley grass appearing to be the preferred host, followed closely by brome grasses (including prairie grass) (Figure 3). Small colonies were sometimes found on wild oat grasses, and alates were occasionally found on Phalaris and Dactylis grasses. Very sparse populations were found on young rice crops. These results are consistent with previous SARDI findings regarding possible host plants in SA (GRDC DAS000170 project), with Bromus species and barley grass being of highest preference of all weed grasses tested.
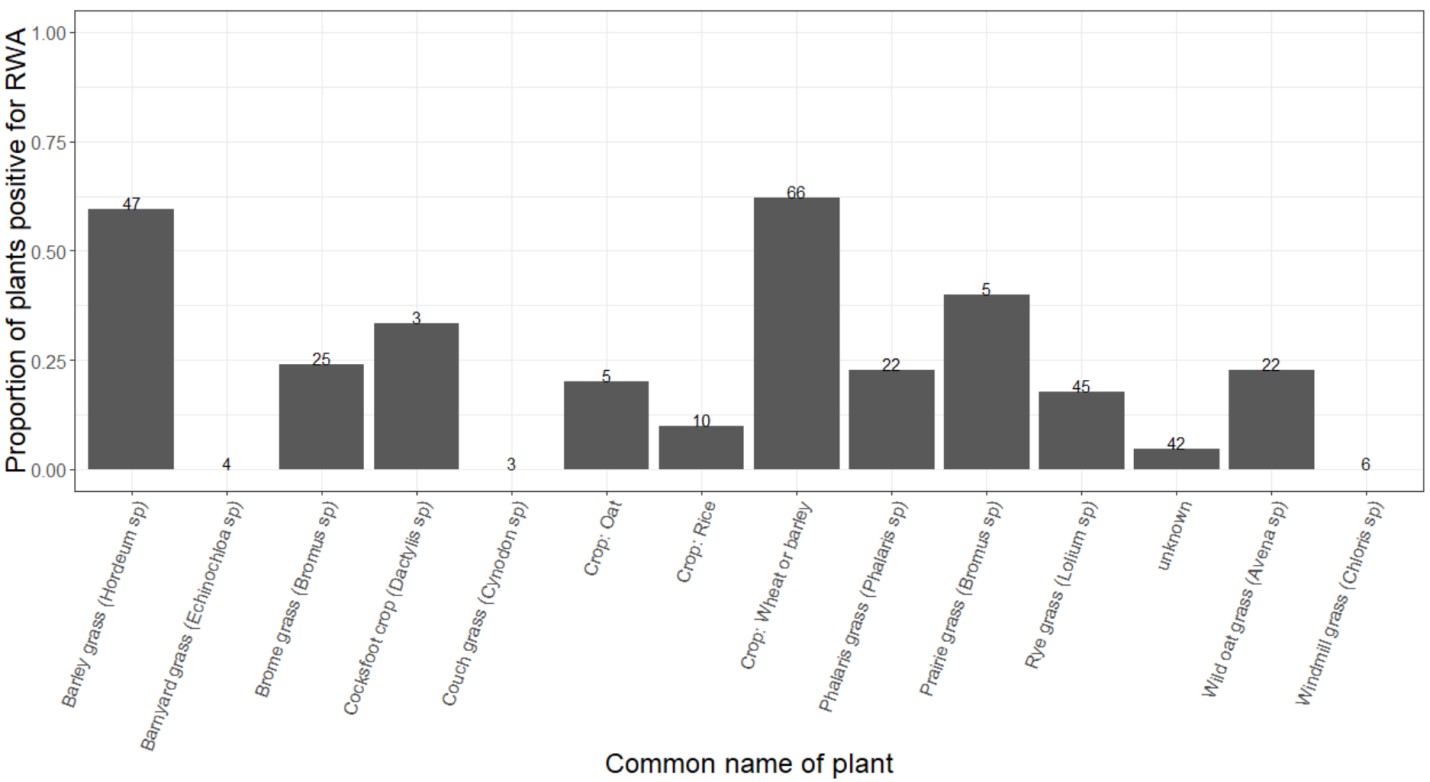
Figure 3. The proportion of individuals within each host plant represented during random plant searches that were positive for RWA (the number of plants searched per species is displayed above the bar). Please note these are preliminary data from Victoria, NSW and Tasmania during spring sampling — formal plant identification is still required for some specimens.
Symptoms such as curled and striped leaves and trapped heads were rarely observed in non-crop grasses, and when they were, they were subtler in appearance than those observed in cereal crops (see Figure 4). Looking for symptoms is therefore not a good strategy for monitoring RWA presence in weeds.

Figure 4. RWA and damage symptoms on barley grass (left), brome (middle) and wheat (right), observed during spring monitoring. The brome grass pictured shows feeding symptoms (striping on the leaf), which was unusual among weeds (Credit: Dr Elia Pirtle, cesar). The wheat shows the striping symptoms that were frequently observed in younger tillers supporting RWA colonies.
During summer, early results suggest host preference has not changed dramatically, however low preference hosts such as Phalaris and Dactylis, which remain green over summer have potentially risen in importance due to a shortage of preferred barley grass and brome hosts (though aphid populations detected on these lower preference hosts remain small as they were in the spring).
Summary
The preliminary summer data collected so far for Victoria and Tasmania suggests that RWA populations have declined dramatically over the summer in the warmer, drier cereal growing regions of Australia. However, despite the population declines, they are still distributed across the whole range of springtime active areas, due to summertime crop irrigation and green reservoirs in towns. Moreover, cooler wetter areas, such as higher elevation areas, may contribute to their over-summering.
Completed and ongoing research
Management options for aphids (GRDC 9176535, CES00004, CES00003)
Cultural management options -RWA (GRDC 9176535)
The preliminary summertime surveillance suggested that little flight activity was occurring in Victoria or SA over summer, and one to two-week old regrowth was observed in cereal paddocks, following rains, showing no colonisation by RWA.
RWA is expected to migrate into cereal paddocks during the seedling stage in early autumn, if good flight conditions are experienced (calm, warm days over 20°C). Therefore, controlling weed hosts, especially those identified as highest preference hosts, before sowing can help reduce starting populations of RWA in and around paddocks.
● Monitoring for the aphid itself on green bridge hosts is advisable, as classic RWA symptoms have been rarely observed on graminaceous species over spring and summer.
● Volunteer cereals and weedy grasses (in particular barley grass and brome grasses) found within next season’s cereal paddocks should be controlled at least 2-3 weeks prior to sowing. This will aid in reducing local numbers of the aphid pre-production.
Cultural management options - GPA
Autumn is the critical time for virus infection in canola, as aphids move from the green bridge into the earliest sown crops. A change to milder, wetter conditions going into autumn may mean that aphid flights are to be expected. This is one aspect of assessing the seasonal risk of virus transmission. Infections can also occur with spring aphid flights, but these probably have little effect on yield.
Indeed, in high risk situations during autumn, (e.g. a significant green bridge, neighbouring clover/medic pasture, weed carryover from fallow) early warning of high levels of aphid infestation is important to minimising virus risk.
● Reducing virus inoculum in the surrounding area is one control. Use herbicides or other tactics to eliminate weed hosts, particularly volunteer canola, wild radish, wild turnip, marshmallow and other broadleaf weeds, which host TuYV and GPA. Ensure paddocks are weed-free for 10–14 days before sowing.
● During autumn flights, GPA alates can look down and detect the contrast between bare earth and green vegetation. This will be a cue for them to fly down and probe the plant and see if it is the type of plant to colonise. It is recommended that you sow into stubble, which will reduce the contrast between bare earth and seedlings.
Chemical management options - RWA (GRDC CES00004)
Seed treatments in cereals are useful for protecting crops against virus transmission at establishment, and with the arrival of RWA in Australia, in providing early season protection against feeding damage. In the recent GRDC investment led by cesar researcher, Lisa Kirkland, the efficacy and length of protection afforded by several insecticide seed treatments against RWA were tested.
The experiment was conducted under ‘semi-field’ conditions using closed artificial microcosms with added aphids (Figure 5). As such, caution should be taken in applying the results of the experiment to field conditions — in closed microcosms watering is abundant and plant roots are limited to the container. Nonetheless, the results have revealed some insightful trends.

Figure 5. Semi-field conditions using closed artificial microcosms (Image credit: cesar).
The seed treatments (shown in Table 1) were tested on wheat (cv Trojan) grown for sixteen weeks. The aphids were introduced two weeks after emergence. Each species was designated its own treatment and aphid populations were counted fortnightly. To simulate aphid colony establishment at different growth stages, after counting, the populations were ‘reset’ back to a level of 30 aphids per microcosm by removing or introducing individuals.
Table 1. Rates and details of chemical treatments examined (Source: Kirkland et al. 2018). Cruiser and Cruiser Opti, products of Syngenta Australia, Sydney; Gaucho, product of Bayer CropScience, Melbourne.
| Product name and treatment level | Active ingredient(s) (g L-1) | Application rate (g a.i. 100 kg-1) | Field rate (mL 100 kg-1) |
|---|---|---|---|
| Untreated | - | - | |
| Cruiser, low | Thiamethoxam 350 | 35 | 100 |
| Cruiser, high | Thiamethoxam 350 | 70 | 200 |
| Cruiser Opti, low | Thiamethoxam 210 + lambda-cyhalothrin 37.5 | 34.7 + 6.2 | 165 |
| Cruiser Opti, high | Thiamethoxam 210 + lambda-cyhalothrin 37.5 | 69.3 + 12.4 | 330 |
| Gaucho, low | Imidacloprid 600 | 72 | 120 |
| Gaucho, high | Imidacloprid 600 | 144 | 240 |
APVMA minor use permits are in place for the use of imidacloprid PER82304 and thiamethoxam PER86231 based seed dressing treatment for the Russian Wheat Aphids in cereals are in winter cereals. Minor use permit can be obtained via this link.
The results of this study are summarised in Figure 6 and show that all insecticide seed treatments currently registered for use in Australian cereal crops are effective against RWA and oat aphid. Specifically, higher label rates of Cruiser® and Cruiser® Opti increased the length of protection by 2-3 weeks in this trial, while the addition of the synthetic pyrethroid lambda-cyhalothrin in Cruiser® Opti did not provide clear benefits over Cruiser® in protection against these aphid species., oat aphid was able to persist and reproduce on wheat at an earlier growth stage than RWA. This indicates the oat aphid is more tolerant to certain insecticides and may therefore re-infest insecticide-treated wheat fields earlier than RWA.
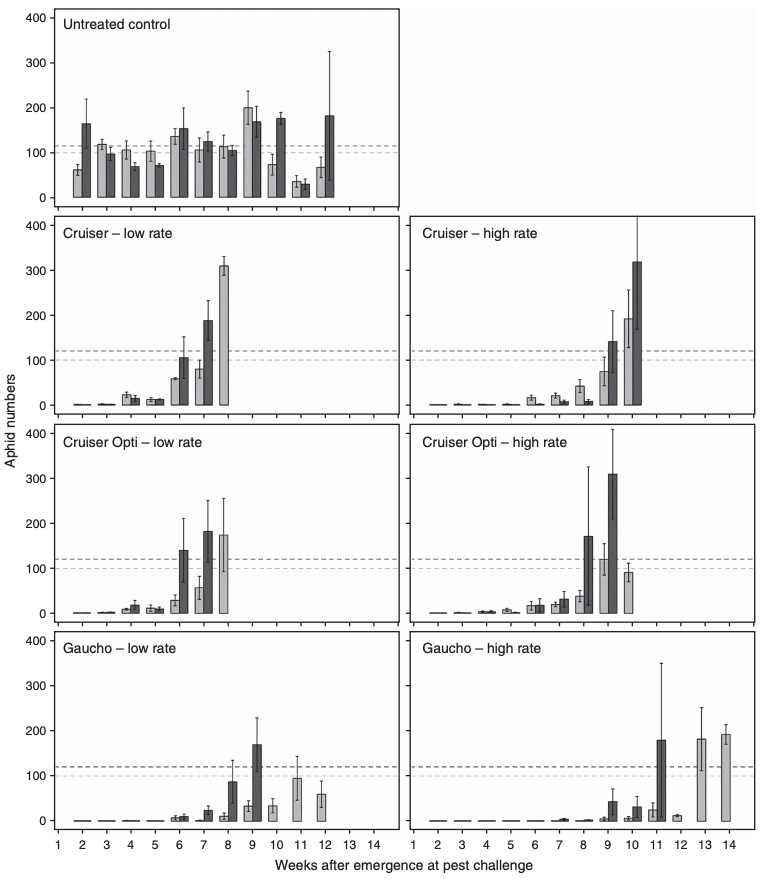
Figure 6. Average numbers of RWA (pale grey bars) and oat aphid (dark grey bars) at weekly intervals after wheat emergence for each chemical treatment. Counts are plotted against the week when aphids were introduced to tubs (Source: Kirkland et al. 2018).
Management thresholds for RWA (GRDC 9176535)
Registered neonicotinoid insecticide seed treatments are very effective to avoid autumn infestation of crops if RWA is migrating. However, over the 2018 season migrations into crops did not occur in most areas where RWA was present, most likely due to unfavourable conditions for aphid survival over summer, and unfavourable flight conditions. Using neonicotinoid seed treatments (imidacloprid, clothianidin, thiamethoxam) on each crop and each rotation can support the evolution of resistance in RWA as seen in other crop pests, such as GPA, which has recently acquired moderate resistance to neonicotinoids in Australia. It is therefore of utmost importance to use neonicotinoid seed treatments judiciously, according to the regional risk, and using the FITE approach.
Currently, only provisional intervention thresholds for RWA are available, which are based on US research (Pike and Alisson, 1991). This research recommends control at the following points: >20% of all plants infested up to GS30 and >10% of tillers infested from late stem elongation (following GS30). Since initial detection of RWA in Australia, growers have been advised to use these thresholds as they represent the best current knowledge.
The current GRDC investment 9176535 is investigating regional economic thresholds for RWA management in Australia. In 2018, 15 trial sites were set up throughout SA, Victoria, NSW and Tasmania by Dr Maarten van Helden and Thomas Heddle (SARDI) in collaboration with regional organisations. Sites were chosen in regions where RWA was known to be established. Cereals tested at each regional trial site included spring wheat and barley. Winter wheat, durum wheat and oats were also tested at some sites. A subset of these trial sites was artificially inoculated with the aphid at a specific time point to ensure thresholds could be developed. This trial site work builds on the SA Grains Industry Trust (SAGIT) Time of Sowing trials conducted by SARDI in 2017 and 2018 in three regions — Bool Lagoon, Roseworthy and Loxton.
Each 2018 trial site included the following treatments: Gaucho® seed treatment, chlorpyrifos treatment, seed treatment plus chlorpyrifos, and no treatment. Yield data were collected for each treatment at each trial. Data on RWA abundance, presence of beneficials and RWA migration times were also collected throughout the season at these sites.
There is currently only one season of trial site data, therefore inferences can yet be made. These trials will be repeated in 2019, which will strengthen our data set and enable further investigation into the relationship between RWA numbers, plant symptoms and yield loss across regions, as well as allowing for development of regional economic thresholds.
Regional threshold trial sites will be run again in 2019 throughout SA, Victoria, Tasmania and NSW. These trial sites will provide two seasons worth of data for the verification of currently used international thresholds, or development of new, regionally specific thresholds.
In the meantime, the following overseas economic thresholds for RWA are recommended:
● >20% of all plants infested up to GS30 and >10% of tillers infested from late stem elongation (GS30 or later).
Management thresholds for GPA (GRDC CES00003)
There are approx. 200 insecticide products registered to control GPA in Australia, but very few of these are available in the grains industry. The most common insecticides used to target GPA in Australian crops are synthetic pyrethroids, organophosphates, carbamates and neonicotinoids. More recently, there has been access to the new chemistry, sulfoxaflor, which is from the 4C mode of action group. GPA has developed resistance to 74 insecticides with different modes of action, as classified by the IRAC. In Australia, there are currently two types of GPA resistance – target site resistance and metabolic resistance.
Pyrethroids and carbamates
Almost all Australian GPA populations tested in recent times are resistant to carbamate and pyrethroid insecticides. Resistance issues have been increasing since 2012, with pyrethroid resistance now found in higher frequencies and in new regions (Figure 7). The same pattern is true for carbamates (Figure 8). Target site resistance is the main type detected in carbamate and pyrethroid resistant GPA, and is the more difficult to manage, as it is an ‘on or off’ type of resistance. If a population is carrying a mutation conferring resistance (modified acetylcholinesterase (MACE) or the kdr or super-kdr mutations), these insecticides will not have an effect on them, because the site where the insecticide is meant to bind, and thereby block an essential insect function, has changed configuration due to a change in the pest DNA. This means the insecticide can no longer bind to the site and block insect functions.
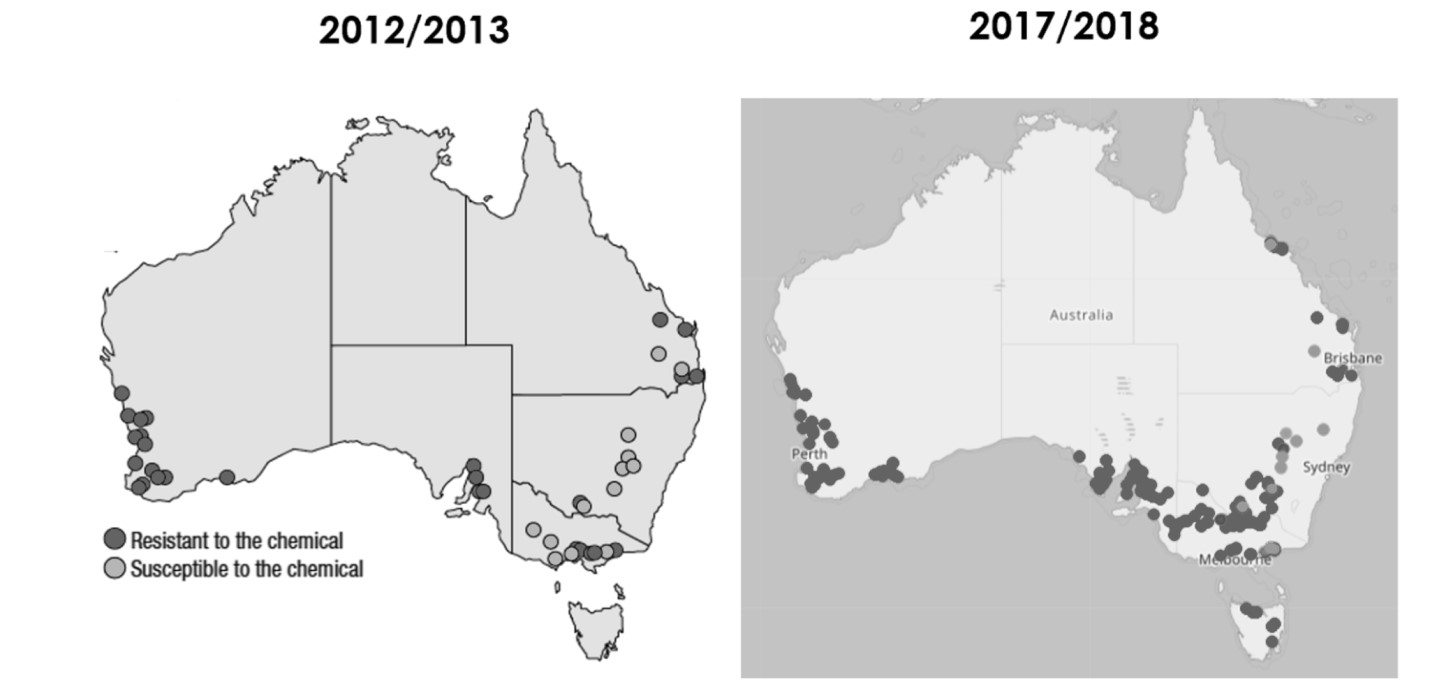
Figure 7. Results of pyrethroid resistance testing in GPA from 2012 to 2018 (dark circles show resistance to the chemical, light circles show susceptibility to the chemical).

Figure 8. Results of carbamate resistance testing in GPA from 2012 to 2018 (dark circles show resistance to the chemical, light circles show susceptibility to the chemical).
Organophosphates and neonicotinoids
In 2012, there was some resistance to organophosphates, and that resistance is now spread widely (Figure 9). In the past 3-4 years, resistance to the neonicotinoids was detected, and again this has unfortunately continued to spread across Australia (Figure 10).

Figure 9. Results of organophosphate resistance testing in GPA from 2012 to 2018 (dark circles show resistance to the chemical, light circles show susceptibility to the chemical).
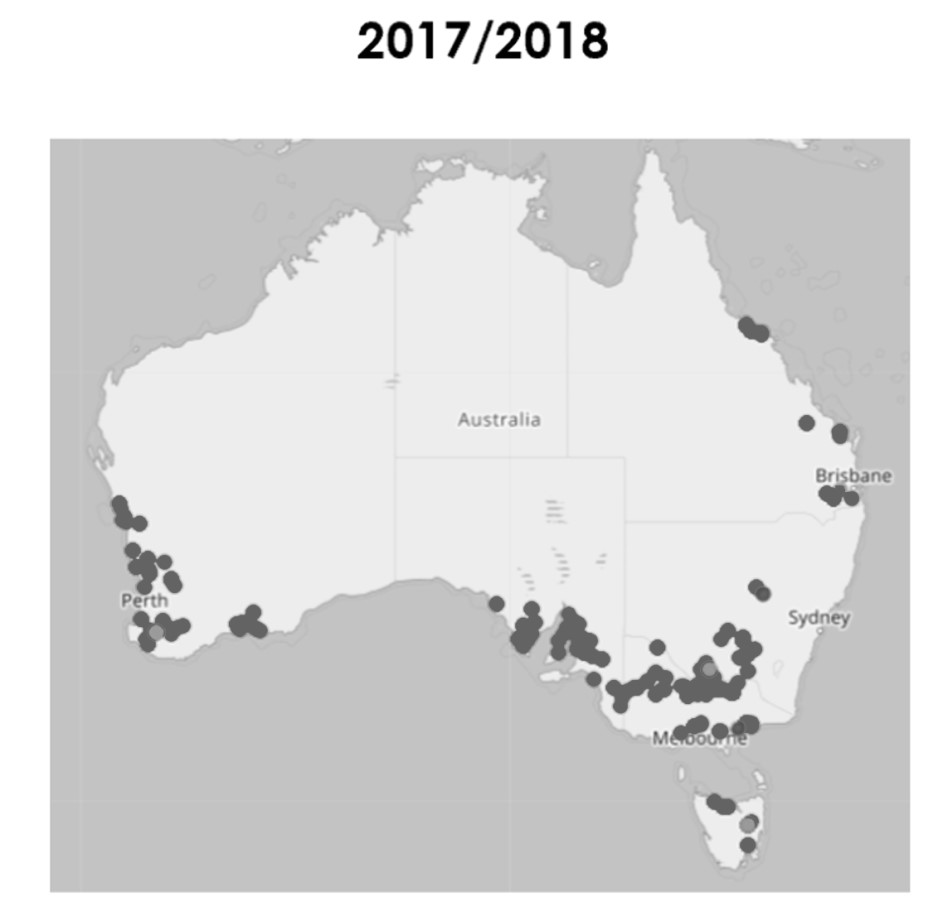
Figure 10. Results of neonicotinoid resistance testing in GPA in 2017 to 2018 (dark circles show resistance to the chemical, light circles show susceptibility to the chemical).
In the case of organophosphate and neonicotinoid resistance, GPA shows metabolic resistance rather than target site. With metabolic resistance, the pest already has some detoxifying enzymes that will help the pest break down the insecticide — they just build up a higher amount of those enzymes and are able to reduce the toxicity of the chemical. It is probably the most common type of resistance as it is using a mechanism that is already naturally at play within the pest.
Future viability of sulfoxaflor
There is another type of neonicotinoid resistance that has showed up in southern Europe, which is a target site resistance. The resistance mutation in this case is called R81T. R81T is particularly concerning because there is cross resistance to sulfoxaflor. There is a trapping program set up to monitor for this particular mutation in Australia. It is important to use a diversity of management practices, and rotate insecticide groups, to prolong the viability of important aphicides such as sulfoxaflor.
Download the Insecticide Resistance Management Strategy (RMS) for GPA
Biological management options
A diverse range of beneficial insects are known to predate on aphids such as GPA and RWA and these populations will build in response to the presence of aphids throughout the season. Growers are encouraged to consider control options that will have minimal impact on beneficial populations.
Parasitic wasps tend to be very good at tracking down aphid populations and moving into a field to feed on them. A good indicator that parasitism is taking place is if aphid mummies are seen on the leaves – they look like golden brown swollen aphids. Other beneficials that also tend to hang out in the canopy and feed on aphids are hoverfly larvae and ladybird beetles, both the larva and the adult. In the case of these generalist beneficials, the adults will lay their eggs amidst aphid populations to give their offspring the best chance of success.
RWA (GRDC 9176535)
GRDC investment 9176535 is investigating beneficial predation of RWA during the summer period, which will add to our knowledge of how to manage RWA at a regional level. Beneficial invertebrates observed actively feeding on RWA populations during spring 2018 surveys included adult and larval ladybird beetles, larval brown lacewings and parasitoid wasps. Other beneficial invertebrates commonly detected around RWA populations included spiders, hoverfly larvae and predatory hemipterans. Large RWA populations frequently showed signs of heavy parasitism by wasps in the form of mummified aphids (Figure 11).

Figure 11. A parasitoid wasp and several RWA mummies on a cereal (Credit: Dr Elia Pirtle, cesar).
Continuation of the green bridge surveillance over 2019/2020 will provide more data regarding key beneficials impacting RWA, and this information may be used to update or develop integrated pest management (IPM) strategies.
Conclusion
Current advice for GPA and RWA management
RWA
● Cultural:
○ Monitoring for the aphid itself on green bridge hosts is advisable, as classic RWA symptoms have been rarely observed on graminaceous species over spring and summer.
○ Volunteer cereals and weedy grasses (in particular barley grass and brome grasses) found within next season’s cereal paddocks should be controlled at least 2-3 weeks prior to sowing. This will aid in reducing local numbers of the aphid pre-production.
● Chemical
○ Registered neonicotinoid insecticide seed treatments are very effective to avoid autumn infestation of crops if RWA is migrating. However, over the 2018 season migrations into crops did not occur in most areas where RWA was present, most likely due to unfavourable conditions for aphid survival over summer, and unfavourable flight conditions.
○ To ensure seed treatments remain a long term viable control option for grains pests, industry stewardship and good resistance management are vital. Growers are urged to use neonicotinoid seed treatments judiciously, according to the regional risk, and using the FITE approach.
○ RWA is easy to detect in autumn and winter before yield is impacted. If RWA is present in potentially damaging numbers, it can be controlled efficiently by insecticide sprays around growth-stages 32-40, eliminating the aphids before there is a risk of yield loss. The overseas threshold is >20% of all plants infested up to GS30 and >10% of tillers infested from late stem elongation (GS30 or later).
● Biological
○ Monitor aphid populations for signs of parasitism, and consider control options that will have minimal impact on beneficial populations. Good information about beneficials can be found in the beneficial back pocket guide, available from the GRDC, or also in the iSPY insect identification manual online. Keep in mind, particularly in the case of transient beneficials such as parasitic wasps, there is likely to be a lag time between when aphid populations are found and when an impact from beneficials is seen as they need time to find the population and build up their own numbers.
GPA
● Cultural
○ Reducing virus inoculum in the surrounding area is one control. Use herbicides or other tactics to eliminate weed hosts, particularly volunteer canola, wild radish, wild turnip, marshmallow and other broadleaf weeds, which host TuYV and GPA. Ensure paddocks are weed-free for 10–14 days before sowing.
○ During autumn flights, GPA can detect the contrast between bare earth and green vegetation. Sowing canola into stubble (where possible) is recommended, which will reduce the contrast between bare earth and emerging seedlings, and thus the chance of aphids infesting new paddocks.
● Chemical
○ It is well recognised that GPA can establish resistance to new chemistries quickly. Following resistance management strategies based on mode of action rotation is of crucial importance in preventing resistance becoming widespread. The mode of action group is listed quite clearly on the label. Follow the available resistance management strategy (RMS) to reduce reliance on single chemical groups, and keep chemical options effective for longer.
○ GPA tends to feed on the older leaves in the lower areas of the canopy. It is important when spraying to ensure effective spray coverage is achieved, use the correct droplet size, correct spray volumes, and always follow the label rate.
● Biological
○ Monitor aphid populations for signs of parasitism, and consider control options that will have minimal impact on beneficial populations. Good information about beneficials can be found in the beneficial back pocket guide, available from the GRDC, or also in the iSPY insect identification manual online. Keep in mind, particularly in the case of transient beneficials such as parasitic wasps, there is likely to be a lag time between when aphid populations are found and when an impact from beneficials is seen as they need time to find the population and build up their own numbers.
Useful resources
To view the RWA Interactive Map
http://www.cesaraustralia.com/sustainable-agriculture/rwa-portal/
GrowNotes Tips & Tactics for Russian Wheat Aphid
https://grdc.com.au/__data/assets/pdf_file/0025/289321/GRDC-Tips-and-Tactics-Russian-Wheat-Aphid.pdf
Russian Wheat Aphid Tactics for Future Control
Russian Wheat Aphid Dynamics in 2017 (research update)
Green peach aphid resistance management strategy
References
Avila, G., Davidson, M., Van Helden, M., and Fagan, L. (2019). The potential distribution of the Russian wheat aphid (Diuraphis noxia): An updated distribution model including irrigation improves model fit for predicting potential spread. Bulletin of Entomological Research, 109(1): 90-101.
Bellati, J., Mangano, P., Umina. P., and Henry., K. (2012). I SPY Insects of Southern Australian Broadacre Farming Systems Identification Manual and Education Resource. Department of Primary Industries and Resources South Australia (PIRSA), the Department of Agriculture and Food Western Australia (DAFWA) and cesar Pty Ltd.
Keating B., A, Carberry., P.S., Hammer., G.L, et al. (2003). An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy, 18: 267–288.
Kirkland, L.S., Pirtle, E.I., & Umina., P.A. (2018). Responses of the Russian wheat aphid (Diuraphis noxia) and bird cherry oat aphid (Rhopalosiphum padi) to insecticide seed treatments in wheat. Crop and Pasture Science 69: 966-973.
Ma., Z., and Bechinski., E.J. (2009). Life tables and demographic statistics of Russian wheat aphid (Hemiptera: Aphididae) reared at different temperatures and on different host plant growth stages. European Journal of Entomology 106:205–210.
Pike KS, and Allison, D. (1991). Russian wheat aphid. Biology, damage and management. Pacific Northwest Cooperative Extension Publication PNW371.
Van Helden, M. (2018). Testing the suitability of Australian native and introduced grasses as host plants for Russian wheat aphid. GRDC DAS000170 SARDI Entomology Report.
Acknowledgements
The research presented here is made possible by the significant contributions of growers through both trial cooperation and the support of the Grains Research and Development Corporation. The authors would like to thank them for their continued support. We would also like to acknowledge other collaborators including Julia Severi (cesar), Anthony van Rooyen (cesar), Dr Siobhan de Little (cesar), Dr Andrew Weeks (cesar), Ary Hoffmann (University of Melbourne), Owain Edwards (CSIRO), Jenny Reidy-Crofts (CSIRO), Matthew Binns (CSIRO), Greg Baker (PIRSA), Maarten van Helden (PIRSA), Tom Heddle (PIRSA), Kym Perry (PIRSA), Ben Congdon (WA DPIRD), Svetlana Micic (DPIRD), and Alan Lord (DPIRD). The authors also acknowledge the assistance of Ken McKee and David Landmeter (Syngenta Australia), Shane Trainer (Bayer Crop Science) and Colin Edmondson (Advanta Seeds).
Project 9176535 is a GRDC investment that seeks to deliver information on RWA management for grain growers. This project is being undertaken by SARDI and cesar. The project team would like to acknowledge 2018 trial site contractors in SA, Victoria, NSW and Tasmania.
Seed treatment research was supported by GRDC and made possible with the support of Advanta Seeds, Syngenta Australia and Bayer CropScience. Untreated Trojan wheat seeds were provided by Advanta Seeds via Longreach Plant Breeders. The authors acknowledge the assistance of Ken McKee, David Landmeter, Shane Trainer, Jenny Reidy-Crofts, Colin Edmondson, and Samantha Ward.
Contact details
Elia Pirtle
cesar Pty Ltd, 293 Royal Parade, Parkville VIC
03 9349 4723
epirtle@cesaraustralia.com
@cesaraustralia
Maarten van Helden
South Australian Research and Development Institute
08 8429 0642
Maarten.VanHelden@sa.gov.au
Jessica Lye
cesar Pty Ltd, 293 Royal Parade, Parkville VIC
03 9349 4723
jlye@cesaraustralia.com
@cesaraustralia
Was this page helpful?
YOUR FEEDBACK
