Yield loss to canola from green peach aphid
Author: Svetlana Micic, Paul Matson, Gerald Skinner, DPIRD | Date: 26 Feb 2019
Key Messages
Green peach aphid colonisation of seedling canola can effect yield and biomass depending on aphid numbers.
Background
Previous work has shown that yield loss caused by green peach aphid (GPA) is complicated by aphid-transmitted virus, such as the turnip yellows virus (see Coutts et al. 2015). It is also common for multiple aphid species to colonise canola at the same time, for example cabbage aphid, which can cause significant yield loss if numbers are high enough. In this study we determined the impact of GPA feeding damage on canola yield and seed quality in the absence of virus and other aphid species.
Aims
Determine yield loss from green peach aphid on seedling canola
Method
Trial 1
Canola cv. GEM was sown into pots on the 25th June 2017 in Albany as per Brennan and Bolland (2007). Six pots, each with 4 plants, were placed into a single aphid proof cage measuring approximately 1m3. A total of 15 cages were used.
Treatments were nil, (no aphids introduced) and aphid introductions at three different growth stages: seedling (3-4 leaf stage); stem elongation and 1% flowering. Aphids were bred under laboratory conditions. Plants in each treatment were uniform in height and growth stage before the addition of aphids.
Aphid populations were measured by counting the number of aphids on 1 randomly selected leaf per plant per pot per tent. The underside of selected leaves were photographed and the number of aphids counted. This was done at three canola growth stages: stem elongation, 50% flowering and podding. Aphid populations were not controlled and plant biomass and yield was measured.
Trial 2
Canola, cv. Bonnito, was dry sown at Katanning on May 22nd 2018. Twenty insect exclusion tents measuring approximately 3m3 were placed over the seeded area to ensure aphids could not colonise the canola from the surrounding area. At the 5 leaf growth stage, canola seedlings were thinned to ensure all tents contained 20 plants in a similar spatial configuration.
Treatments included a nil, (no aphids introduced), nil sprays (aphids introduced, no chemical control); aphids controlled at raceme development and aphids controlled at 10% flowering. Sulfoxaflor or a paraffinic oil was used to control aphids. Treatments were replicated 5 times.
Aphid introductions were done at the 5 leaf stage by placing a green leaf with 10 live aphids onto each plant within the insect proof tents.
Aphid populations in the tents were measured by counting the number of aphids on 10 random plant leaves. This was done three times during the growing season (27 Aug, 17 Sept, 8 Oct). Aphid populations were left to either persist on plants until harvest or were controlled with an insecticide. Aphids found in control tents were sprayed. Plants were assessed for biomass, yield and seed quality.
Results
Trial 1
The timing of aphid introduction and subsequent aphid numbers had a measurable impact on dry matter weight, pods per plant and yield. Early aphid introduction resulted in the highest aphid numbers (Fig. 1) and plants with the least biomass and number of pods (Table 1). The introduction of aphids post stem elongation also caused a significant decrease in pod number (Table 1).
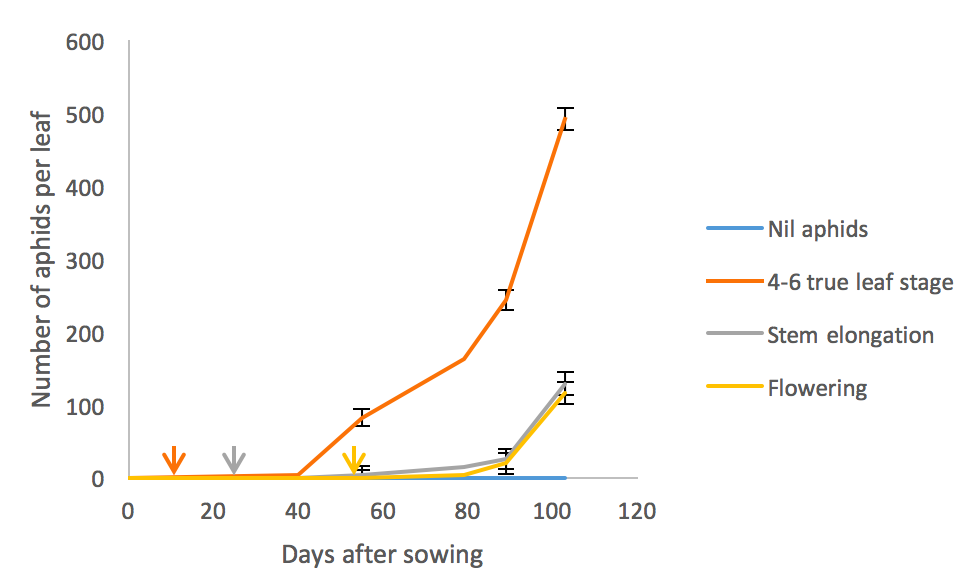 Figure 1. Green peach aphid population dynamics from different dates of introductions ± standard error (SE). Arrows indicate the timing of aphid introductions relative to sowing date.
Figure 1. Green peach aphid population dynamics from different dates of introductions ± standard error (SE). Arrows indicate the timing of aphid introductions relative to sowing date.
Table 1. Average canola yield components per plant for the green peach aphid treatments, letters indicate significance within treatments at P<0.001.
Treatment |
Plant weight (g) |
Pods per plant |
|---|---|---|
No aphids | 25a | 195a |
Flowering | 20b | 64b |
Stem elongation | 20b | 21c |
Seedling | 11c | 0d |
The temperature in the glasshouse could not be controlled and was 22°C - 30°C leading to ideal conditions for aphid populations to increase (Liu and Meng 1999). Aphids were observed to migrate from leaves and onto stems.
Trial 2
Aphid numbers peaked in mid-September (Figure 2) with an average of 800 aphids per 10 leaves in the nil spray treatment. By early November canola treatments without aphid control had senesced earlier than treatments with control. If aphids were controlled at raceme development or at 10% flowering there was no yield penalty (Table 2). There was no significant difference in moisture percent, oil percent or protein content in seed from the various treatments.
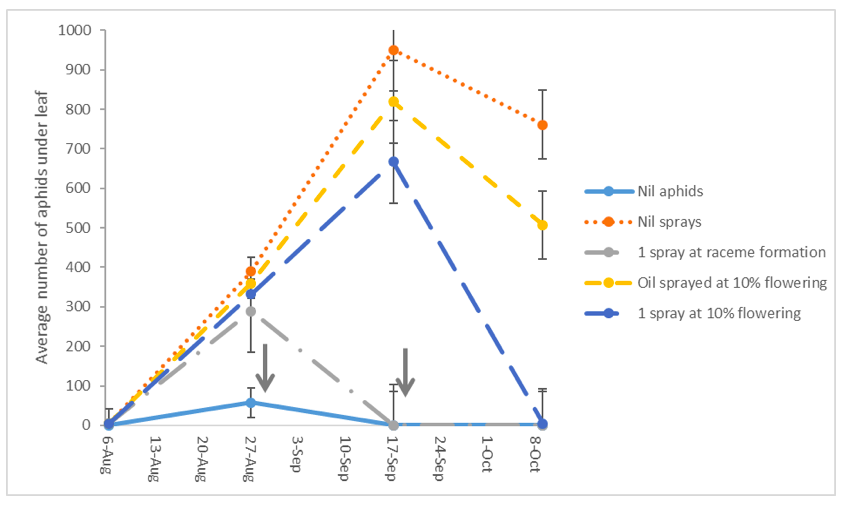 Figure 2. Green peach aphid population dynamics from a single introduction at canola at 5 leaf stage ± standard error (SE). Arrows indicate dates of spray applications
Figure 2. Green peach aphid population dynamics from a single introduction at canola at 5 leaf stage ± standard error (SE). Arrows indicate dates of spray applications
Table 2. Average canola yield components per plant for the green peach aphid treatments, letters indicate significant differences (P<0.001)
Treatment | Number of pods | Seed weight (g) | Stubble weight (g) |
|---|---|---|---|
Nil aphids | 307c | 34.3c | 44.7b |
Nil sprays | 72a | 0.3a | 13.7a |
1 spray at raceme formation | 294c | 27.2c | 36b |
Oil sprayed at 10% flowering | 129b | 4.5a | 20.7a |
1 spray at 10% flowering | 340c | 33.5c | 45.2b |
Conclusion
The glasshouse trial shows that canola seedlings are not able to compensate, for feeding damage by GPA if aphid populations continue to increase at their optimal development rate even if soil moisture stress is not a factor.
The field trial suggests that if GPA colonise canola at the seedling stage and are not controlled leading to more than 600 aphids on leaves by 10% flowering canola plants will incur a yield loss. It is however possible that the yield penalty in commercial crops will not be as large as the trial did not take into consideration the patchy nature of aphid colonisation, aphid predators that can cause a significant reduction in aphids.
References
Brennan, RF. and MD. Bolland, 2007: Comparing the potassium requirements of canola and wheat. Australian Journal of Agricultural Research 58, 359–366.
Coutts, B., R. Jones, P. Umina, J. Davidson, G. Baker and M. Aftab, 2015: Beet western yellows virus (synonym: Turnip yellows virus) and green peach aphid in canola. Available at: Beet Western Yellows Virus (Synonym: Turnip Yellows Virus) And Green Peach Aphid In Canola
Liu, S. and X. Meng, 1999: Modelling development time of Myzus persicae (Hemiptera: Aphidae) at constant and natural temperatures. Bulletin of Entomological Research. 89, 53-63.
Murray, D., M. Clarke, D. Ronning, 2013: The current and potential costs of invertebrate pests in grain crops.
Acknowledgments
The research undertaken is made possible by funding from Boosting Grains.
Was this page helpful?
YOUR FEEDBACK
