Phytoplasma in grain legumes: what we know / don’t know
Take home messages
- Phytoplasma has caused significant losses in grain legume crops, is widespread in the northern region and infects a wide range of hosts
- Many things remain unknown. A leafhopper vector is suspected but not confirmed
- Monitor surrounding weeds for phytoplasma symptoms and maintain good farm hygiene to reduce risk of spread into crops
- Growers should monitor crops for leafhopper activity and phytoplasma infection and report any outbreaks to Qld DAF entomologists and/or pathologists.
In recent years there have been outbreaks of phytoplasma in a number of grain legume crops throughout the northern region. Phytoplasma are specialised bacteria that infect the phloem cells of plants and are spread from plant to plant by insect vectors, such as leaf hoppers. Symptoms are variable and depend on the crop type and timing of infection. In severely affected plants, which are infected prior to flowering, symptoms generally include stunting with masses of small cupped leaves (little leaf) that either do not flower or display phyllody - when flower structures turn green and leafy and pods are either not present or are sterile (Figure 1). Plants infected after flowering often have normal growth lower on the plant, followed by masses of deformed flowers and small pods that remain green, fail to produce harvestable seed or have damaged seed.
Phytoplasma disease outbreaks have been common and widespread in mung bean (Vigna radiata)crops and other crops including soybeans (Glycine max), peanuts (Arachis hypogaea) and pigeon pea (Canjanus cajan), both in the late spring plantings of 2016, and in early 2017 plantings. Mung bean crops were affected in all major production areas in early 2017 from locations such as Ayr, Capella, Kingaroy, Dalby, St George, Inverell, Gurley, Wee Waa and Narrabri - spanning a distance of over 1,200 km from north to south (Figure 2). Many mung bean crops in the Dalby region had greater than 40 % disease incidence and up to 70 % from a crop in Capella in central Queensland. Losses were estimated at $800,000 in that season. At the same time, phytoplasma outbreaks also occurred in horticultural crops from several locations in eastern Australia. Although we have found phytoplasma in a wide range of weed hosts, we do not yet know if one or more of these are critical in the disease cycle that leads to disease epidemics in nearby crops.
A devastating disorder of several soybean crops from the Cecil Plains region in late autumn 2016 was also associated with phytoplasma infection. Affected plants produced no, or very few filled pods, and instead, had a proliferation of tiny immature pods as shown in Figure 3. Affected plants also remained green while unaffected crops nearby matured and browned off as expected. Significant losses were sustained by the growers with almost 100% of plants affected in some paddocks on a couple of farms, with virtually no yield and estimated losses of $400,000 on each farm.
We are continuing to confirm associations of phytoplasma infection with unusual pod and seed symptoms. It appears that strange fruit symptoms such as puffy pod in mungbeans (Figure 4) or shrivelled, discoloured seeds in peanuts (Figure 5) and chickpeas (Figure 6) are associated with phytoplasma infection. To date, 49 peanut plants with shrivelled kernels (typical of “peanut kernel shrivel - PKS” disorder) have all tested positive for phytoplasma and have also had some degree of leaf symptoms typical of phytoplasma. While there may be additional causes of shrivelled kernels in peanuts, our testing has not yet found similar peanut plants (i.e. with shrivelled kernels) without phytoplasma infection. Losses due to PKS have been estimated by the Peanut Company of Australia to be in the order of $1 million during the worst years. Also, a loss of confidence among Bundaberg peanut growers due to PKS has limited new peanut expansion in recent years, which represents an opportunity cost of several million dollars.
A similar story is emerging for puffy pod symptoms in mung beans with all 13 plants tested so far with typical puffy pod symptoms, testing positive for phytoplasma. Our testing will need to be done over more than one season and from various locations to increase our confidence that phytoplasma is strongly linked to these fruit symptoms, but also to investigate if there may be other possible causes in the absence of phytoplasma.
There are many different phytoplasmas that can affect crops. Therefore, we aimed to determine the identification of the phytoplasma present. We also determined the geographical extent of disease and the host range, as this may provide evidence of hot spots of disease and possible associations to key weed hosts surrounding crops. To investigate the identification and diversity of phytoplasmas present we studied the P1/P7 region of the 16S gene from more than 23 crop and weed hosts from a wide geographical range from far north QLD to northern NSW. Partial genome sequence indicates there are two species of phytoplasma in crops and weeds, Candidatus Phytoplasma aurantifolia and C. Phytoplasma australiense. The vast majority of samples were from the C. Phytoplasma aurantifolia group which has been previously reported in Australia from pigeon pea (Canjanus cajan) and stylosanthes.
A known vector of phytoplasma, the phloem-feeding brown leaf hopper (Orosius orientalis, Figure 7), was collected from some affected mung bean crops but it is not certain that it was associated with these disease outbreaks. We are currently developing and validating rapid molecular assays to identify potential vector species and determine if they are carrying the phytoplasma pathogen. However, at this stage we have not confirmed the insect vector responsible for transmission of phytoplasma into the affected crops.
To our knowledge, these have been the most significant, widespread outbreaks of phytoplasma in broadacre crops to occur in this region of Australia. It is unclear what the underlying reasons are for this sudden increase in disease incidence in recent years. In 2018, the incidence of disease did reduce compared to previous years, but was still present in most summer legume crops inspected. Prior to the succession of outbreaks in recent years, phytoplasma infection is estimated to have only occurred on average at a very low incidence (usually much less than 1%) and often not at all in most crops. One reason for the great increase in disease in some seasons could be that spring rains favour weeds that carry the phytoplasma and host the putative vector (leafhopper). It is also possible that the phytoplasma may be vectored by another insect (most likely within the Cicadellidae family).
There was a mild, wetter winter in 2016 followed by a dry spring. We suspect that these conditions were favourable for the phytoplasma and vector to survive through winter on weeds and then move into nearby spring-planted mungbean crops as the nearby weeds dried out. Further research and paying close attention to any new infestations is vital to gain a better understanding of the disease and its insect vector and develop strategies to minimise damage. We are continuing further studies to determine: the diversity of phytoplasma across crop and weed hosts and across seasons, their geographical range, which insect species are vectors, and possible management options. Monitoring surrounding weeds for phytoplasma symptoms and maintaining good farm hygiene may reduce the spread into crops.
It is currently unclear if controlling leafhoppers with an insecticide would prevent the transmission of phytoplasma. Previous experience from other insect-vectored diseases would indicate that spraying was an ineffective management technique, because transmission of the disease usually occurs early in the crop growth, and at very low vector densities – often below the level of easy detection with traditional sampling methods. Infected leafhoppers are also likely to be moving into crops from surrounding weeds (perhaps from some distance away), so spraying in-crop may have little effect to stop transmission, as they probably only need to feed for a short period to cause infection. Attempting to control the vectors through the application of multiple prophylactic sprays of a non-selective insecticide (e.g. synthetic pyrethroids, organophosphates) will also run the risk of flaring other pests like thrips, Helicoverpa, silverleaf whitefly and mites. In addition, every application of SPs and OPs selects for resistance in Helicoverpa.
Growers should monitor crops for leafhopper activity and phytoplasma infection and report any outbreaks to Qld DAF entomologists and pathologists.

Figure 1. Phytoplasma symptoms on mung bean plants often include masses of small cupped leaves (little leaf) and phyllody - when flower structures turn green and leafy and pods are either not present or are sterile. Early infected plants are often stunted. Later infected plants may have puffy pod symptoms
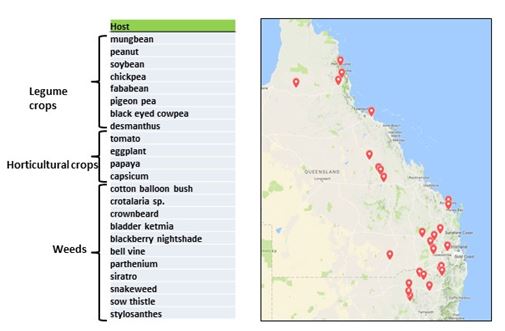
Figure 2. Map of collection sites in Northern Region for phytoplasma from crop and weed hosts. Range of hosts confirmed by molecular assay for phytoplasma

Figure 3. Soybean crop with close to 100 % incidence of phytoplasma. Symptoms included a proliferation of tiny, sterile pods; thickened and curled leaves, and prolonged greening of crop

Figure 4. Symptoms of puffy pod on mung bean include swollen, soft pods with a green mottled (net-like) appearance on exterior of pods that contain brown, deformed seeds.

Figure 5. Kernel symptoms on phytoplasma-infected peanuts (left) compared to healthy kernels (right). Symptoms were typical of peanut kernel shrivel disorder and included shrivelled away from outer shell with obviously raised, pronounced veins on kernels which may be from pinkish in colour for immature pods to dark on mature pods, and swollen funiculus on a shrivelled kernel

Figure 6. Late infected chickpea with shrivelled and marked seeds. Later flowers also display phyllody (flowers become sterile green leaf-like structures)

Figure 7. A known vector of phytoplasma, the brown leafhopper (Orosius orientalis) has been found in affected crops but remains unconfirmed as the vector involved in recent disease outbreaks
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC (projects DAQ00186 and DAN00202), the authors would like to thank them for their continued support.
Contact details
Murray Sharman
Principal Plant Pathologist
Department of Agriculture and Fisheries
Ecosciences Precinct, GPO Box 267, Brisbane 4001
Mb: 0467 721 400
Email: murray.sharman@daf.qld.gov.au
Hugh Brier
Principal Entomologist
Department of Agriculture and Fisheries
PO Box 23, Kingaroy, 4610
Mb: 0428 188 069
Email: hugh.brier@daf.qld.gov.au
GRDC codes:
DAQ00186 – Improving grower surveillance, management, epidemiology, knowledge and tools to manage crop disease
DAN00202 – New tools and germplasm for Australian pulse and oil seed breeding programs to respond to changing virus threats
Was this page helpful?
YOUR FEEDBACK
